1. A perfect gas undergoes isothermal compression, which reduces its volume by 2.20 dm³. The final pressure and volume of the gas are 5.04 bar and 4.65 dm³, respectively. Calculate the original pressure of the gas in (i) bar, (ii) atm. A vessel of volume 22 1 dm3 contains 2.0 mol H and 1.0 mol N at 273 15 K initially AII
1. A perfect gas undergoes isothermal compression, which reduces its volume by 2.20 dm³. The final pressure and volume of the gas are 5.04 bar and 4.65 dm³, respectively. Calculate the original pressure of the gas in (i) bar, (ii) atm. A vessel of volume 22 1 dm3 contains 2.0 mol H and 1.0 mol N at 273 15 K initially AII
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.127QP: A 1.000-g sample of an unknown gas at 0C gives the following data: P(atm) V (L) 0.2500 3.1908 0.5000...
Related questions
Question
I need help with my homework. Please show me the calculation.
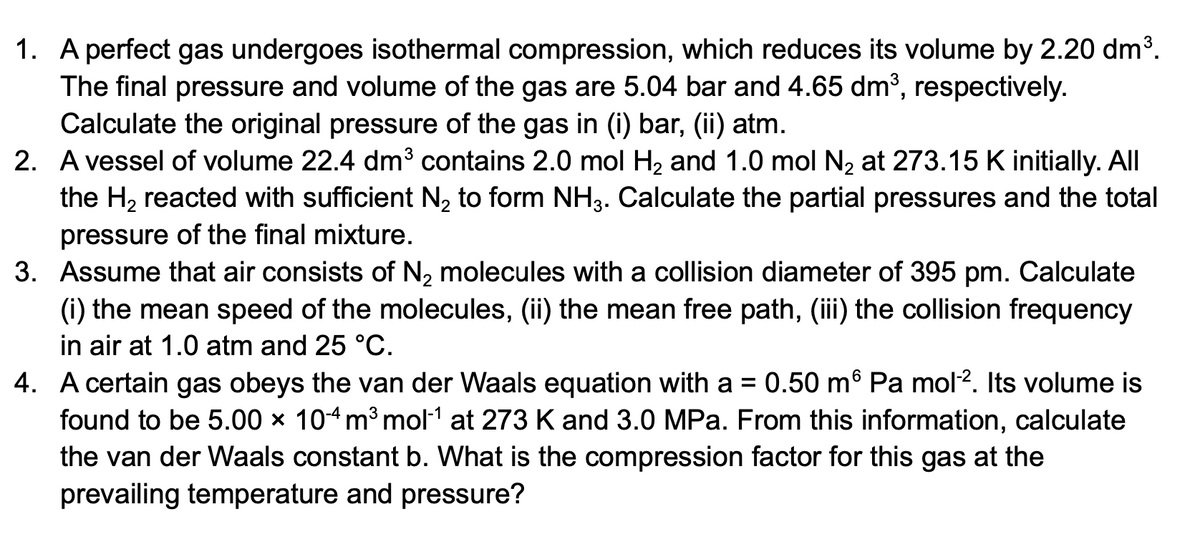
Transcribed Image Text:1. A perfect gas undergoes isothermal compression, which reduces its volume by 2.20 dm³.
The final pressure and volume of the gas are 5.04 bar and 4.65 dm³, respectively.
Calculate the original pressure of the gas in (i) bar, (ii) atm.
2. A vessel of volume 22.4 dm³ contains 2.0 mol H2 and 1.0 mol N, at 273.15 K initially. All
the H2 reacted with sufficient N, to form NH3. Calculate the partial pressures and the total
pressure of the final mixture.
3. Assume that air consists of N2 molecules with a collision diameter of 395 pm. Calculate
(i) the mean speed of the molecules, (ii) the mean free path, (ii) the collision frequency
in air at 1.0 atm and 25 °C.
0.50 m® Pa mol2. Its volume is
4. A certain gas obeys the van der Waals equation with a
found to be 5.00 × 104m³ mol1 at 273 K and 3.0 MPa. From this information, calculate
the van der Waals constant b. What is the compression factor for this gas at the
prevailing temperature and pressure?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning