1. Calculate the concentration (mol/L or M) of calcium ions in each water sample using the molar ratio between the calcium ions and EDTA in Reaction 1. 2. Determine the water hardness of your sample by converting the calcium ion concentration (mol/L) to ppm CaCO3. You will need to use the molar mass of CaCO3 to convert the concentration to units of mg/L. Then you can convert your concentration from mg/L to parts per million (ppm) using the conversion factor: 1mg/L = 1ppm. 3. Use Equation 1 to calculate the average calcium ion concentration and Equation 2 and 3 to determine the standard deviation of three titration trials. Average (x) = Σ.χ. n or x = sum of all values to be averaged number of terms to be averaged Equation 1
1. Calculate the concentration (mol/L or M) of calcium ions in each water sample using the molar ratio between the calcium ions and EDTA in Reaction 1. 2. Determine the water hardness of your sample by converting the calcium ion concentration (mol/L) to ppm CaCO3. You will need to use the molar mass of CaCO3 to convert the concentration to units of mg/L. Then you can convert your concentration from mg/L to parts per million (ppm) using the conversion factor: 1mg/L = 1ppm. 3. Use Equation 1 to calculate the average calcium ion concentration and Equation 2 and 3 to determine the standard deviation of three titration trials. Average (x) = Σ.χ. n or x = sum of all values to be averaged number of terms to be averaged Equation 1
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter28: Determination Of The Hardness Of Water
Section: Chapter Questions
Problem 3ASA: A 100-mL sample of hard water is titrated with the EDTA solution in Problem 2. The same amount of...
Related questions
Question
The volume of water sample= 15mL of hard water
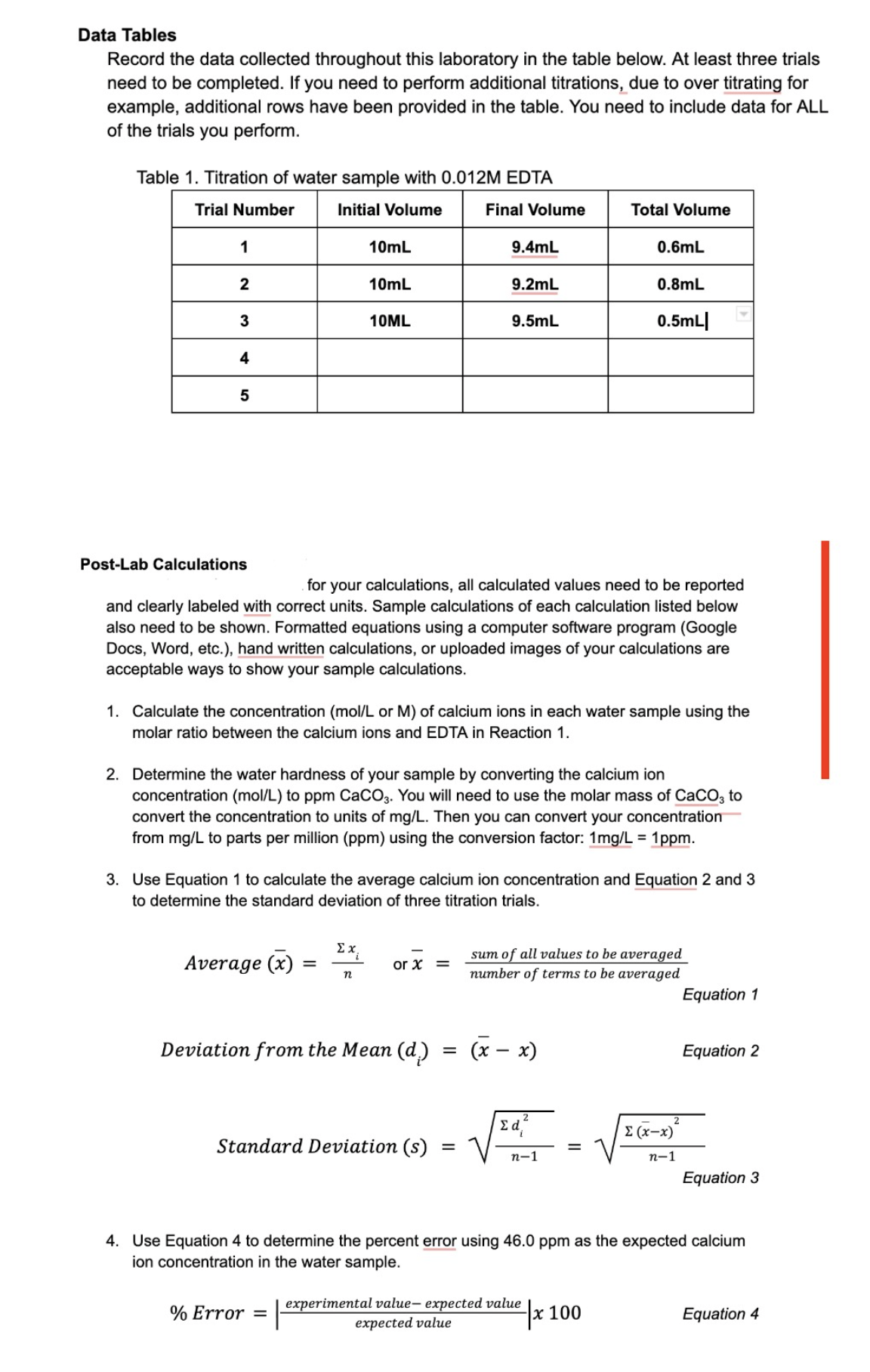
Transcribed Image Text:Data Tables
Record the data collected throughout this laboratory in the table below. At least three trials
need to be completed. If you need to perform additional titrations, due to over titrating for
example, additional rows have been provided in the table. You need to include data for ALL
of the trials you perform.
Table 1. Titration of water sample with 0.012M EDTA
Trial Number
Initial Volume
1
2
3
4
5
Post-Lab Calculations
10mL
Average (x) =
10mL
10ML
Ex
n
for your calculations, all calculated values need to be reported
and clearly labeled with correct units. Sample calculations of each calculation listed below
also need to be shown. Formatted equations using a computer software program (Google
Docs, Word, etc.), hand written calculations, or uploaded images of your calculations are
acceptable ways to show your sample calculations.
% Error =
1. Calculate the concentration (mol/L or M) of calcium ions in each water sample using the
molar ratio between the calcium ions and EDTA in Reaction 1.
2. Determine the water hardness of your sample by converting the calcium ion
concentration (mol/L) to ppm CaCO3. You will need to use the molar mass of CaCO3 to
convert the concentration to units of mg/L. Then you can convert your concentration
from mg/L to parts per million (ppm) using the conversion factor: 1mg/L = 1ppm.
3. Use Equation 1 to calculate the average calcium ion concentration and Equation 2 and 3
to determine the standard deviation of three titration trials.
or X =
Deviation from the Mean (d)
Final Volume
Standard Deviation (s)
9.4mL
9.2mL
9.5mL
=
=
(x − x)
-
Total Volume
2
ed²
0.6mL
sum of all values to be averaged
number of terms to be averaged
n-1
0.8mL
0.5mL
=
experimental value- expected value
expected value
x 100
Σ (x-x)²
n-1
Equation 1
4. Use Equation 4 to determine the percent error using 46.0 ppm as the expected calcium
ion concentration in the water sample.
Equation 2
Equation 3
Equation 4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning