1. Circle the skeletal structure that corresponds to the following condensed formula; (CH3)2CHCH₂CH(OH)CCl₂CH3 DO CI CI SIST OH OOH XOT POST C OH OH ASSIST CI CHTA POST OST-DO NOT POST SHIST-00 2. How are the structures in the following pair related? - DO NOT POST DO NOT ASSIST DO NOT POT-DO NOT ST NOT POST POST-DO and DOLNOT Pos A. They are isomers B. They are resonance structures C. They are identical structures D. They are completed unrelated; they have different molecular formulas SIST-DO NO 3. Indicate the hybridization of the nitrogen ion in each compound below. MACE CEN-H N POST-DO A. 1-sp²; 2-sp³ B. 1-sp; 2-sp³ C. 1-sp³; 2-sp D. 1-sp; 2-sp² محمد 2 388 888888888
1. Circle the skeletal structure that corresponds to the following condensed formula; (CH3)2CHCH₂CH(OH)CCl₂CH3 DO CI CI SIST OH OOH XOT POST C OH OH ASSIST CI CHTA POST OST-DO NOT POST SHIST-00 2. How are the structures in the following pair related? - DO NOT POST DO NOT ASSIST DO NOT POT-DO NOT ST NOT POST POST-DO and DOLNOT Pos A. They are isomers B. They are resonance structures C. They are identical structures D. They are completed unrelated; they have different molecular formulas SIST-DO NO 3. Indicate the hybridization of the nitrogen ion in each compound below. MACE CEN-H N POST-DO A. 1-sp²; 2-sp³ B. 1-sp; 2-sp³ C. 1-sp³; 2-sp D. 1-sp; 2-sp² محمد 2 388 888888888
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter6: Alkanes & Alkenes
Section: Chapter Questions
Problem 10CTQ: Consider the Newman projection below. a. Draw a full Lewis structure of this molecule with...
Related questions
Question
100%
please answer all if possible please!
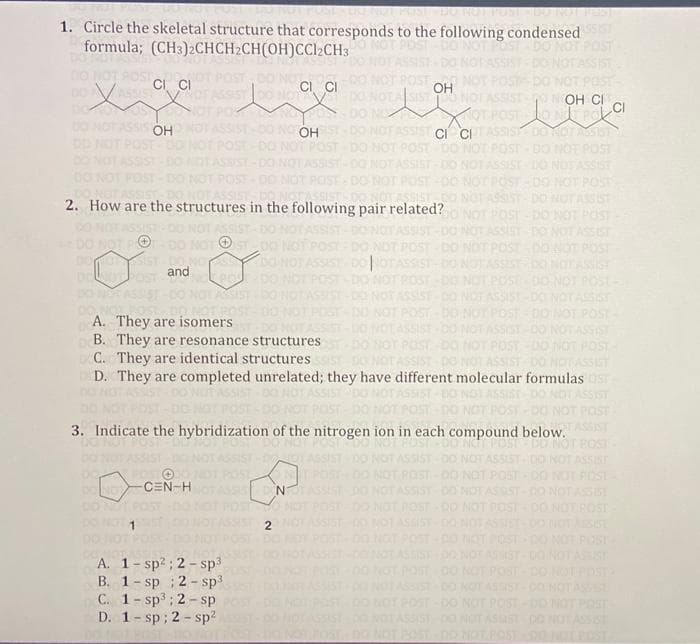
Transcribed Image Text:POST DO NOTIPUNI IDUTNO
1. Circle the skeletal structure that corresponds to the following condensed
formula; (CH3)2CHCH2CH(OH)CCl2CH3
CI CI
CI CI
xige live Lov
SSIST DO NOT
OH
SISTO
POST-DO NOT POST
NOT ASSIST OOH CI
NOT POST CONTROL CI
DO NO
ASSIS OH
SSIST-00 NO OH
NOT ASSIST CI CI
POST
DO NOT ASSIST
OST-DO NOT POST
86153
ASSIST
2. How are the structures in the following pair related?
POST-DO NOT POST
ASSIST- DO NOT ASSIST
POST - DO NOT POST
ASSIST-DO NOT ASSIST. DO NOT ASSIST
POST-DO NOT POST - DO NOT POST
DO NOT POT-DO
DOMONSIST-DO
and
BY
SSIST DO NOTASSIST-DO NOT ASSIST-D
DO NOT POST-DO NOT POST-DO NOT POST-DO NOT POST-
SSIST
ASSIST
SSIST
POST
POST
A. They are isomers
ASSIST
B. They are resonance structures.
C. They are identical structures
DO NOT ASSIST
ASSIST
D. They are completed unrelated; they have different molecular formulas OST
3. Indicate the hybridization of the nitrogen ion in each compound below.
DD NOT
NOT ASSIS
DO NOV -CEN-HI
NOT
NOT POST-DO NOT POST DO NOT POST
NOT ASSIST DO NOT ASSIST-DO NOT ASSIST
DO NOT POST - DO NOT POST
ASSIST
A. 1-sp²; 2-sp³
B. 1-sp; 2-sp³
C. 1-sp³; 2-sp
D. 1-sp; 2-sp²
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning