1. Circle the volumes of the titrated NaOH(aq) which you want to use for your calculations. How and why did you choose these volumes of NaOH(aq)? 2. What is phenolphthalein and why is it required in this experiment? 3. What type(s) of reactions occurred in this titration? 4. (a) The sodium ion did not take part in this chemical reaction. What do we call such an ion? (b) Draw a simple diagram which shows how the sodium ion mixes with water in solution. What do we call this physical process?
1. Circle the volumes of the titrated NaOH(aq) which you want to use for your calculations. How and why did you choose these volumes of NaOH(aq)? 2. What is phenolphthalein and why is it required in this experiment? 3. What type(s) of reactions occurred in this titration? 4. (a) The sodium ion did not take part in this chemical reaction. What do we call such an ion? (b) Draw a simple diagram which shows how the sodium ion mixes with water in solution. What do we call this physical process?
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 1P
Related questions
Question
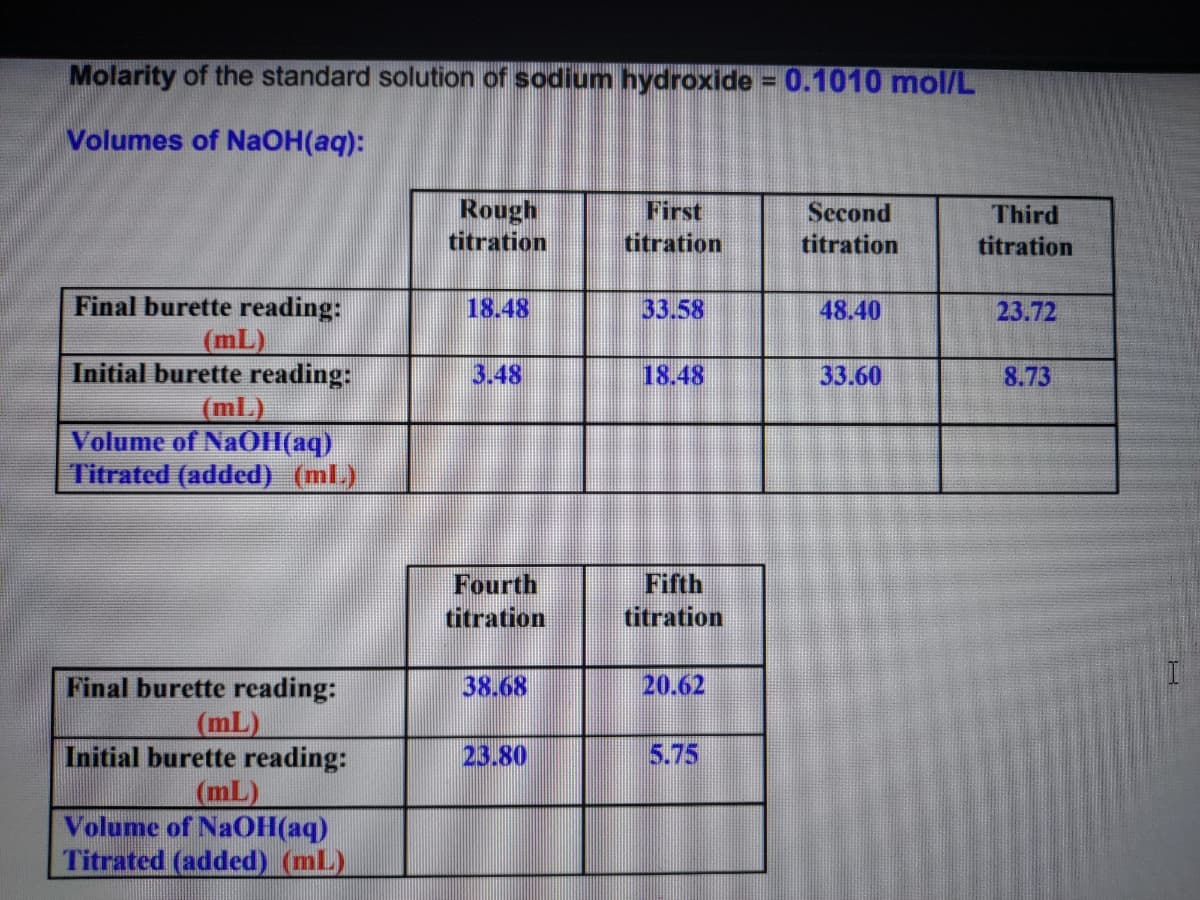
Transcribed Image Text:Molarity of the standard solution of sodium hydroxide = 0.1010 mol/L
%3D
Volumes of NaOH(aq):
Rough
titration
First
Second
Third
titration
titration
titration
Final burette reading:
18.48
33.58
48.40
23.72
(mL)
Initial burette reading:
3.48
18.48
33.60
8.73
(mL)
Volume of NaOH(aq)
Titrated (added) (ml.)
Fourth
titration
Fifth
titration
38.68
20.62
Final burette reading:
(mL)
Initial burette reading:
(mL)
Volume of NaOH(aq)
Titrated (added) (mL)
23.80
5.75
Transcribed Image Text:1. Circle the volumes of the titrated NaOH(aq) which you want to use for your calculations.
How and why did you choose these volumes of NaOH(aq)?
2. What is phenolphthalein and why is it required in this experiment?
3. What type(s) of reactions occurred in this titration?
4. (a) The sodium ion did not take part in this chemical reaction. What do we call such an ion?
(b) Draw a simple diagram which shows how the sodium ion mixes with water in solution.
What do we call this physical process?
PREDATOR
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning