1. Determination of heat capacity of calorimeter: 50.0 mL. H2O at the initial temperature of 23.5 °C is added to a calorimeter (coffee cup) and stirred and kept for 5 minutes. Then 50.0 mL. of hot water at the temperature of 98.0 °C is added to the calorimeter contained the 50.0 mL. H2O at 23.5 °C. The temperature of the mixture is recorded every 30 second, the following data is obtained:
1. Determination of heat capacity of calorimeter: 50.0 mL. H2O at the initial temperature of 23.5 °C is added to a calorimeter (coffee cup) and stirred and kept for 5 minutes. Then 50.0 mL. of hot water at the temperature of 98.0 °C is added to the calorimeter contained the 50.0 mL. H2O at 23.5 °C. The temperature of the mixture is recorded every 30 second, the following data is obtained:
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.83E: Benzoic acid, C6H5COOH, is a common standard used in bomb calorimeters, which maintain a constant...
Related questions
Question

Transcribed Image Text:Hint: This is the diluted : heat capacity = 3.184 J / g °C and D = 1.00 g/ mole
Heat of reaction = - (heat of solution) - heat of calorimetry
c. Calculate the heat of reaction for the following chemical reaction:
NaOH (s) + HC1(aq) --------→ NaC(aq) + H2O(1)
Using the following reactions:
NaOH(s) -
-→ NaOH(aq).
AH = -102 kj / mole
NaOH(aq) + HCl(aq) --
calculations.
-→ NaCl(aq) + H2O(1). use the heat of reaction from your
- -
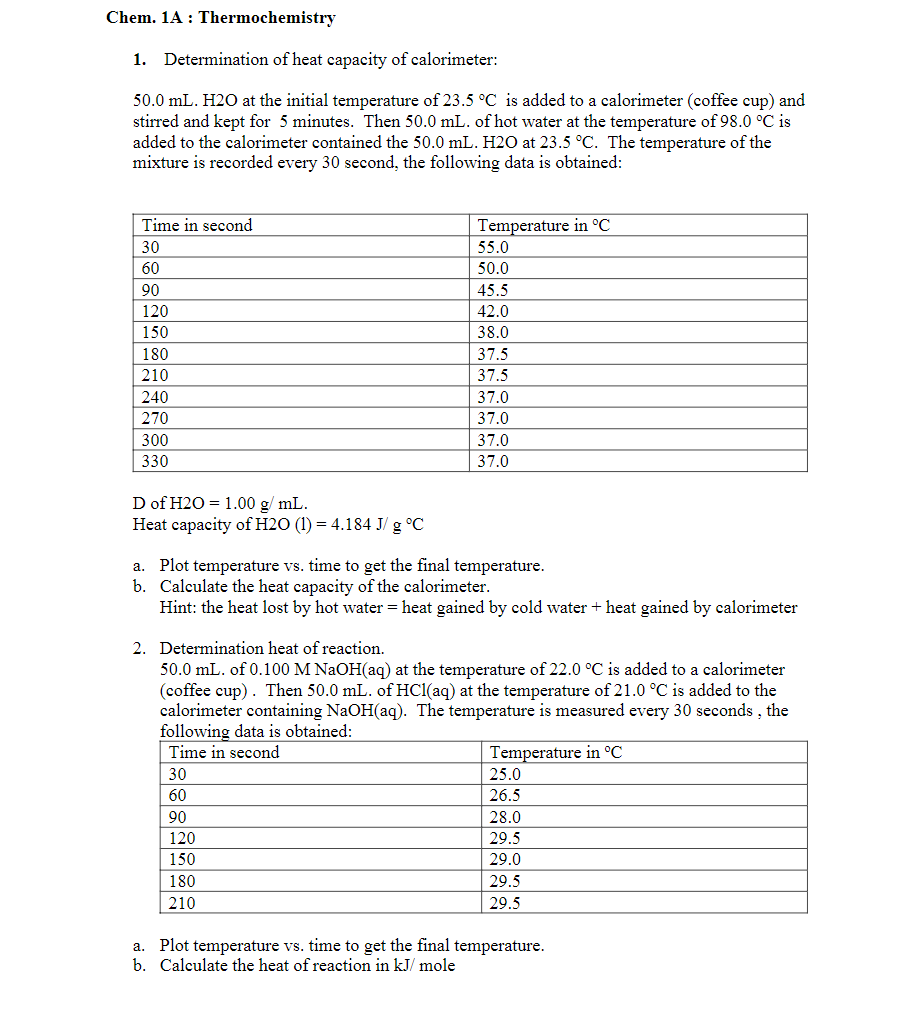
Transcribed Image Text:Chem. 1A : Thermochemistry
1. Determination of heat capacity of calorimeter:
50.0 mL. H2O at the initial temperature of 23.5 °C is added to a calorimeter (coffee cup) and
stirred and kept for 5 minutes. Then 50.0 mL. of hot water at the temperature of 98.0 °C is
added to the calorimeter contained the 50.0 mL. H2O at 23.5 °C. The temperature of the
mixture is recorded every 30 second, the following data is obtained:
Time in second
Temperature in °C
30
55.0
60
50.0
90
45.5
120
42.0
150
38.0
180
37.5
210
37.5
240
37.0
270
37.0
300
37.0
330
37.0
D of H2O = 1.00 g/ mL.
Heat capacity of H2O (1) = 4.184 J/ g °C
a. Plot temperature vs. time to get the final temperature.
b. Calculate the heat capacity of the calorimeter.
Hint: the heat lost by hot water = heat gained by cold water + heat gained by calorimeter
2. Determination heat of reaction.
50.0 mL. of 0.100 M NaOH(aq) at the temperature of 22.0 °C is added to a calorimeter
(coffee cup). Then 50.0 mL. of HCl(aq) at the temperature of 21.0 °C is added to the
calorimeter containing NaOH(aq). The temperature is measured every 30 seconds , the
following data is obtained:
Time in second
Temperature in °C
30
25.0
60
26.5
90
28.0
120
29.5
150
29.0
180
29.5
210
29.5
a. Plot temperature vs. time to get the final temperature.
b. Calculate the heat of reaction in kJ/ mole
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning