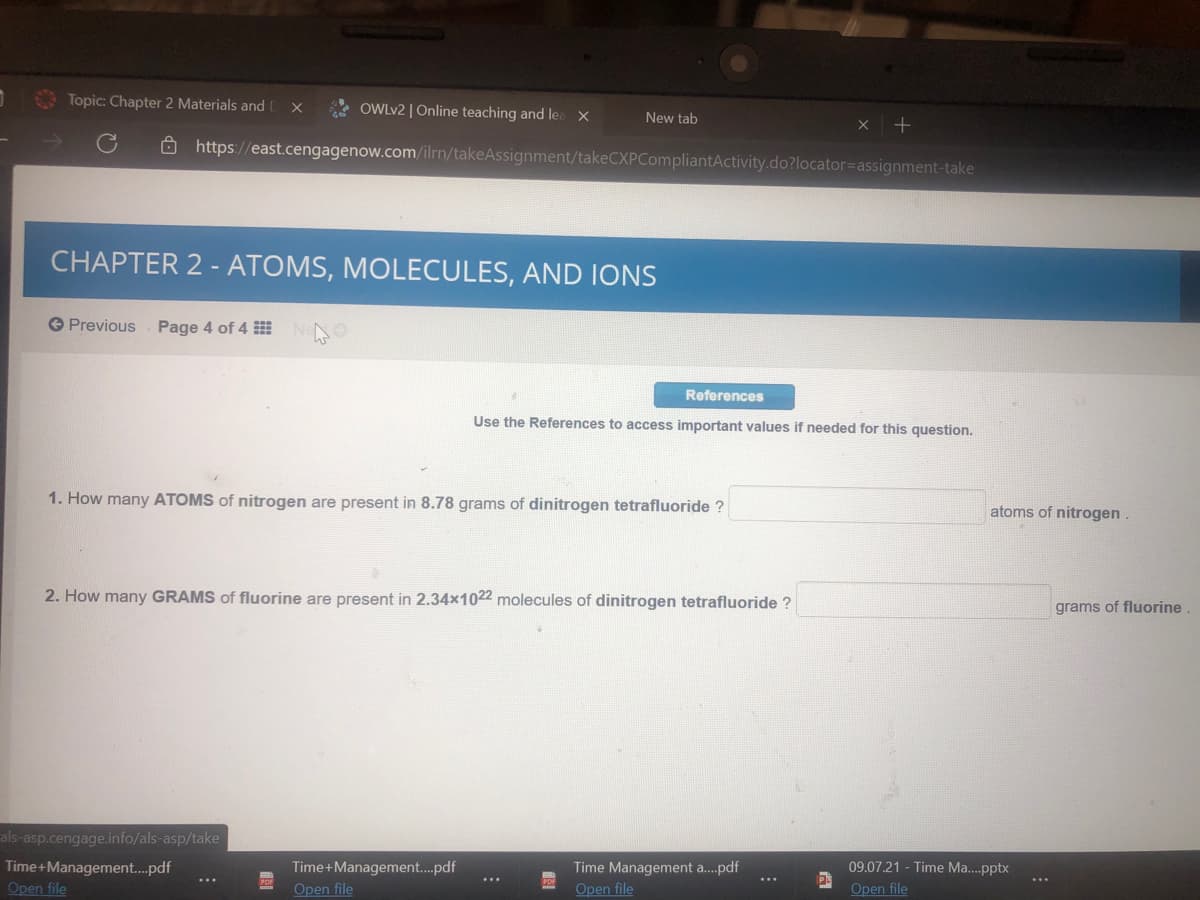
1. How many ATOMS of nitrogen are present in 8.78 grams of dinitrogen tetrafluoride ? atoms of nitrogen . 2. How many GRAMS of fluorine are present in 2.34x1022 molecules of dinitrogen tetrafluoride ? grams of fluorine
1. How many ATOMS of nitrogen are present in 8.78 grams of dinitrogen tetrafluoride ? atoms of nitrogen . 2. How many GRAMS of fluorine are present in 2.34x1022 molecules of dinitrogen tetrafluoride ? grams of fluorine
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 26P: Following the pattern of Figure 6.21, work out the correlation diagram for the BeN molecule, showing...
Related questions
Question
Transcribed Image Text:Topic: Chapter 2 Materials and
* OWLV2 | Online teaching and lea
New tab
https://east.cengagenow.com/ilrn/takeAssignment/takeCXPCompliantActivity.do?locator=Dassignment-take
CHAPTER 2 - ATOMS, MOLECULES, AND IONS
O Previous
Page 4 of 4
References
Use the References to access important values if needed for this question.
1. How many ATOMS of nitrogen are present in 8.78 grams of dinitrogen tetrafluoride ?
atoms of nitrogen.
2. How many GRAMS of fluorine are present in 2.34x1022 molecules of dinitrogen tetrafluoride ?
grams of fluorine
als-asp.cengage.info/als-asp/take
Time+Management.pdf
Open file
Time+Management.pdf
Time Management a.pdf
09.07.21 - Time Ma..pptx
...
Open file
Open file
Open file
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning