Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter10: Quantity Relationships In Chemical Reactions
Section: Chapter Questions
Problem 70E: Classify each of the following statements as true or false: a Coefficients in a chemical equation...
Related questions
Question
Can you please help me with question 1.
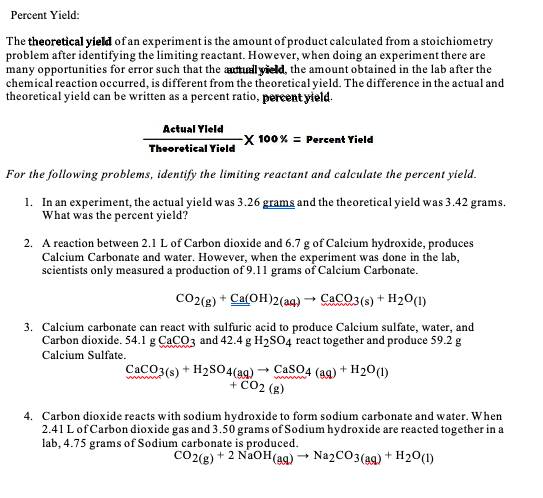
Transcribed Image Text:Percent Yield:
The theoretical yield of an experiment is the amount of product calculated from a stoichiometry
problem after identifying the limiting reactant. However, when doing an experiment there are
many opportunities for error such that the adtuall yield, the amount obtained in the lab after the
chemical reaction occurred, is different from the theoretical yield. The difference in the actual and
theoretical yield can be written as a percent ratio, percent yield.
Actual Yleld
-X 100% = Percent Yield
Theoretical Yield
For the following problems, identify the limiting reactant and calculate the percent yield.
1. In an experiment, the actual yield was 3.26 grams and the theoretical yield was 3.42 grams.
What was the percent yield?
2. A reaction between 2.1 L of Carbon dioxide and 6.7 g of Calcium hydroxide, produces
Calcium Carbonate and water. However, when the experiment was done in the lab,
scientists only measured a production of 9.11 grams of Calcium Carbonate.
co2(g) + Ca(OH)2(aq) → CaCO3(s) + H20(1)
3. Calcium carbonate can react with sulfuric acid to produce Calcium sulfate, water, and
Carbon dioxide. 54.1 g CaCO3 and 42.4 g H2SO4 react together and produce 59.2 g
Calcium Sulfate.
CaCO3(s) + H2S04(a9) → CasO4 (ag) + H2O(1)
+ CO2 (g)
ww w
4. Carbon dioxide reacts with sodium hydroxide to form sodium carbonate and water. When
2.41 L of Carbon dioxide gas and 3.50 grams of Sodium hydroxide are reacted together in a
lab, 4.75 grams of Sodium carbonate is produced.
CO2(g) + 2 NaOH(a9) → Na2CO3(a9) + H2O(1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning
