1. In the hypochlorite oxidation of cyclohexanol to cyclohexanone, what purpose does the acetie-acid serve? HC -1:fhnnf the carhon ateme in the 3C enectra
1. In the hypochlorite oxidation of cyclohexanol to cyclohexanone, what purpose does the acetie-acid serve? HC -1:fhnnf the carhon ateme in the 3C enectra
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter8: Haloalkanes, Halogenation, And Radical Reactions
Section8.7: Radical Autoxidation
Problem AQ: Linoleic acid is shown below. What makes this fatty acid particularly susceptible to autoxidation?...
Related questions
Question
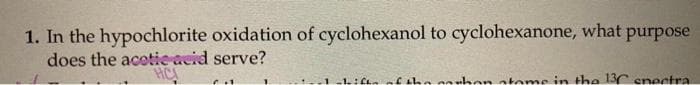
Transcribed Image Text:1. In the hypochlorite oxidation of cyclohexanol to cyclohexanone, what purpose
does the acetieacid serve?
HCA
-1ifhnnf the carhon ateme in the 3C snectra
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning