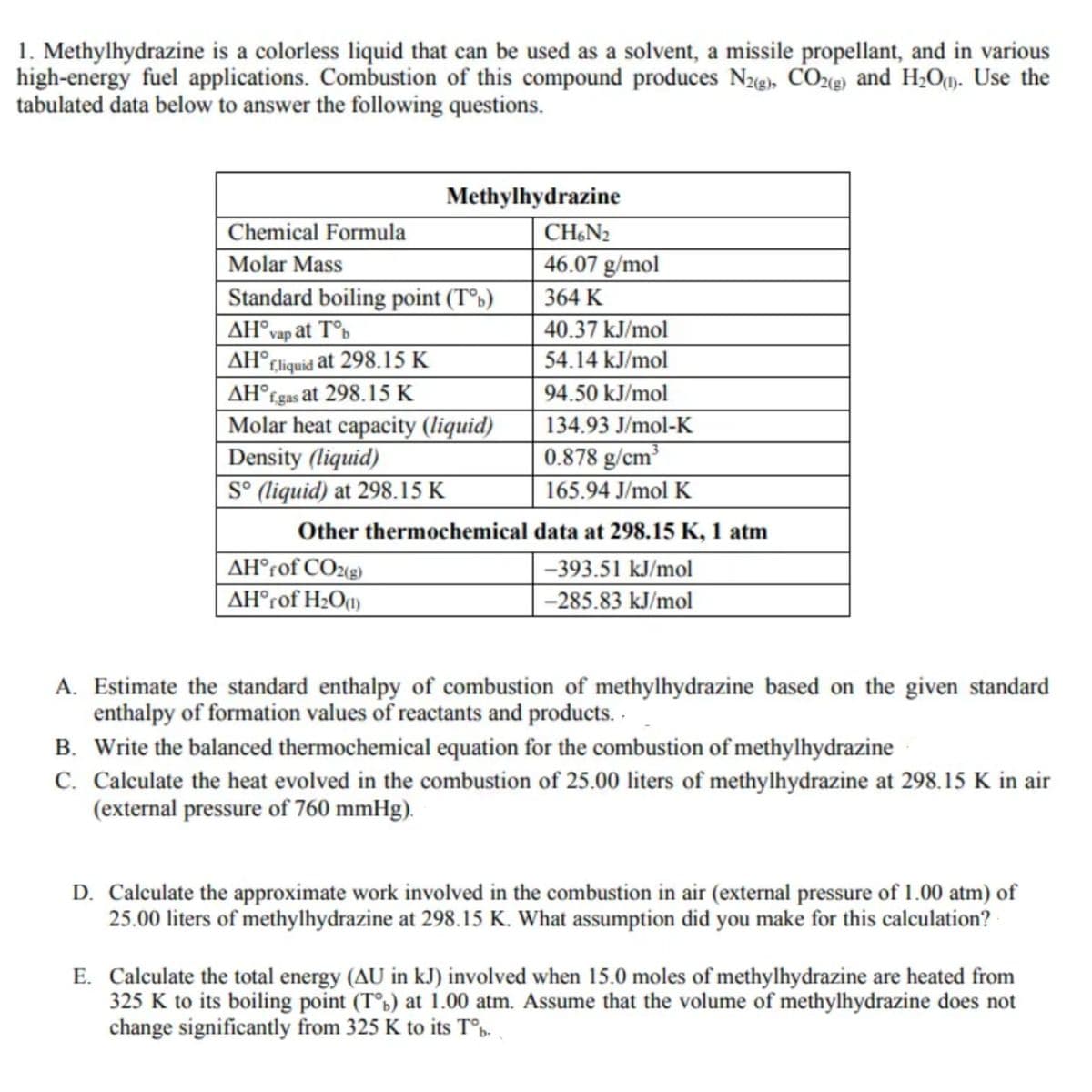
1. Methylhydrazine is a colorless liquid that can be used as a solvent, a missile propellant, and in various high-energy fuel applications. Combustion of this compound produces Nz, COe) and H;Ou). Use the tabulated data below to answer the following questions. Methylhydrazine Chemical Formula CH&N2 Molar Mass 46.07 g/mol Standard boiling point (T°%) AH°vap at Tºb AH°fliquid at 298.15 K fgas at 298.15 K Molar heat capacity (liquid) Density (liquid) S° (liquid) at 298.15 K 364 K 40.37 kJ/mol 54.14 kJ/mol AH°i 94.50 kJ/mol 134.93 J/mol-K 0.878 g/cm 165.94 J/mol K Other thermochemical data at 298.15 K, 1 atm |AH°rof CO2«g) AH°rof H2Ou) -393.51 kJ/mol -285.83 kJ/mol A. Estimate the standard enthalpy of combustion of methylhydrazine based on the given standard enthalpy of formation values of reactants and products. . B. Write the balanced thermochemical equation for the combustion of methylhydrazine C. Calculate the heat evolved in the combustion of 25.00 liters of methylhydrazine at 298.15 K in air (external pressure of 760 mmHg).
Analyzing Infrared Spectra
The electromagnetic radiation or frequency is classified into radio-waves, micro-waves, infrared, visible, ultraviolet, X-rays and gamma rays. The infrared spectra emission refers to the portion between the visible and the microwave areas of electromagnetic spectrum. This spectral area is usually divided into three parts, near infrared (14,290 – 4000 cm-1), mid infrared (4000 – 400 cm-1), and far infrared (700 – 200 cm-1), respectively. The number set is the number of the wave (cm-1).
IR Spectrum Of Cyclohexanone
It is the analysis of the structure of cyclohexaone using IR data interpretation.
IR Spectrum Of Anisole
Interpretation of anisole using IR spectrum obtained from IR analysis.
IR Spectroscopy
Infrared (IR) or vibrational spectroscopy is a method used for analyzing the particle's vibratory transformations. This is one of the very popular spectroscopic approaches employed by inorganic as well as organic laboratories because it is helpful in evaluating and distinguishing the frameworks of the molecules. The infra-red spectroscopy process or procedure is carried out using a tool called an infrared spectrometer to obtain an infrared spectral (or spectrophotometer).
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images









