1. Might any of the ratios you calculated for Table 2 above be useful for predicting how the freezing point of a solution would differ from that of the pure liquid? Explain.
1. Might any of the ratios you calculated for Table 2 above be useful for predicting how the freezing point of a solution would differ from that of the pure liquid? Explain.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 106SCQ
Related questions
Question
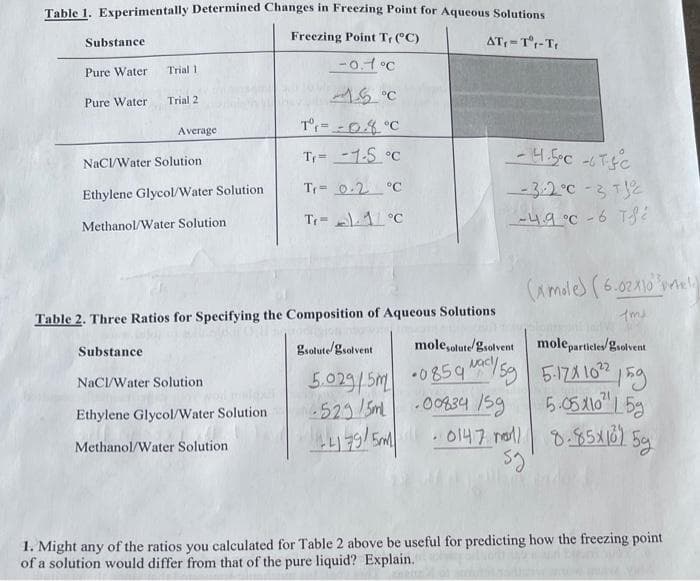
Transcribed Image Text:Table 1. Experimentally Determined Changes in Freezing Point for Aqueous Solutions
Substance
Freezing Point Tr (°C)
AT = T°r-T
Trial 1
-0.1°C
Pure Water
Pure Water
Trial 2
T=-04°C
Average
Tr= -1-5 °C
NaCI/Water Solution
Ethylene Glycol/Water Solution
Tr= 0.2 °C
-3:2°C - 3 T2
Tr- A°C
-49°C - 6 TS
Methanol/Water Solution
(Amoles (6.02X10
Table 2. Three Ratios for Specifying the Composition of Aqueous Solutions
gsolute/gsolvent
mole,olutegsolvent
moleparticles/gsolvent
Substance
5.029/5M •085q /sg 59
5-121 102
NaCI/Water Solution
5.05X10" L 5g
. 0147 na 0.85X16) 5g
.0434 /59
523/5m
5M
Ethylene Glycol/Water Solution
Methanol/Water Solution
1. Might any of the ratios you calculated for Table 2 above be useful for predicting how the freezing point
of a solution would differ from that of the pure liquid? Explain.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax