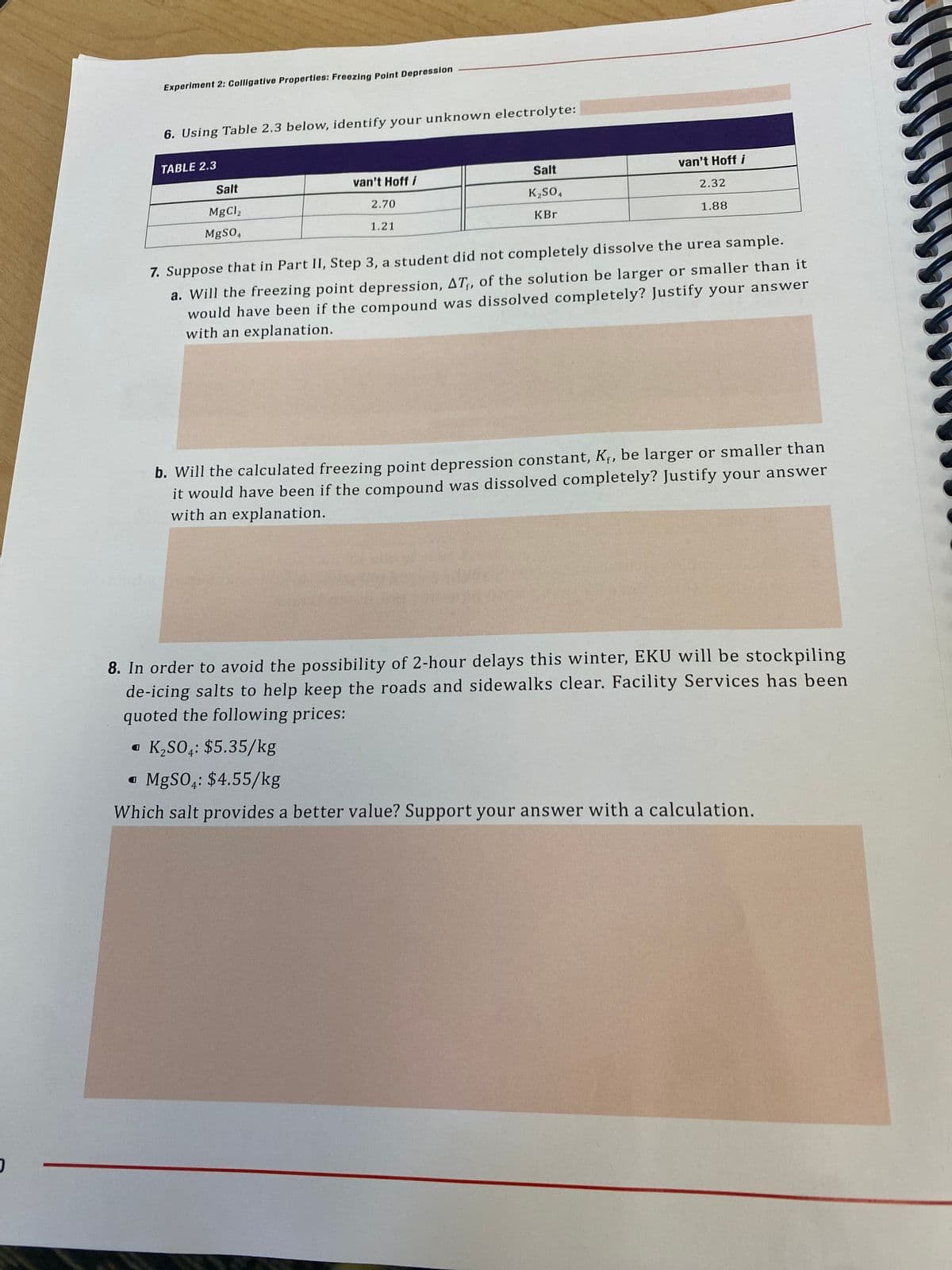
6. Using Table 2.3 below, identify your unknown electrolyte: TABLE 2.3 Salt van't Hoff i Salt van't Hoff i K,so, 2.32 MgCl, 2.70 KBr 1.88 MgSO, 1.21 7. Suppose that in Part II, Step 3, a student did not completely dissolve the urea sample. a. Will the freezing point depression, AT, of the solution be larger or smaller than it would have been if the compound was dissolved completely? Justify your answer with an explanation. b. Will the calculated freezing point depression constant, Kf, be larger or smaller than it would have been if the compound was dissolved completely? Justify your answer with an explanation. 8. In order to avoid the possibility of 2-hour delays this winter, EKU will be stockpiling de-icing salts to help keep the roads and sidewalks clear. Facility Services has been quoted the following prices: • K,SO;: $5.35/kg • MgSO,: $4.55/kg Which salt provides a better value? Support your answer with a calculation.
6. Using Table 2.3 below, identify your unknown electrolyte: TABLE 2.3 Salt van't Hoff i Salt van't Hoff i K,so, 2.32 MgCl, 2.70 KBr 1.88 MgSO, 1.21 7. Suppose that in Part II, Step 3, a student did not completely dissolve the urea sample. a. Will the freezing point depression, AT, of the solution be larger or smaller than it would have been if the compound was dissolved completely? Justify your answer with an explanation. b. Will the calculated freezing point depression constant, Kf, be larger or smaller than it would have been if the compound was dissolved completely? Justify your answer with an explanation. 8. In order to avoid the possibility of 2-hour delays this winter, EKU will be stockpiling de-icing salts to help keep the roads and sidewalks clear. Facility Services has been quoted the following prices: • K,SO;: $5.35/kg • MgSO,: $4.55/kg Which salt provides a better value? Support your answer with a calculation.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 7RQ
Related questions
Question
Question 8.
Transcribed Image Text:Experiment 2: Colligative Propertles: Freezing Point Depression
6. Using Table 2.3 below, identify your unknown electrolyte:
TABLE 2.3
Salt
van't Hoff i
Salt
van't Hoff i
2.32
K,SO,
MgCl,
2.70
KBr
1.88
MgSO,
1.21
7. Suppose that in Part II, Step 3, a student did not completely dissolve the urea sample.
a. Will the freezing point depression, AT,, of the solution be larger or smaller than it
would have been if the compound was dissolved completely? Justify your answer
with an explanation.
b. Will the calculated freezing point depression constant, Kf, be larger or smaller than
it would have been if the compound was dissolved completely? Justify your answer
with an explanation.
8. In order to avoid the possibility of 2-hour delays this winter, EKU will be stockpiling
de-icing salts to help keep the roads and sidewalks clear. Facility Services has been
quoted the following prices:
• K,SO,: $5.35/kg
• MGSO,: $4.55/kg
Which salt provides a better value? Support your answer with a calculation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning