1. Molecular Weight = 44; with 1 0 INFRARED SPECTRUM 0.8 0.6 0.4 0.2 2000 Wavenumber (cm-1) 3000 1000 2. Molecular Weight: 113; with 1 N (all other atoms are C and H) 0.8 0.4 2000 Wavenumber (cm-1) 3000 1000 Transmitance Transmitance
1. Molecular Weight = 44; with 1 0 INFRARED SPECTRUM 0.8 0.6 0.4 0.2 2000 Wavenumber (cm-1) 3000 1000 2. Molecular Weight: 113; with 1 N (all other atoms are C and H) 0.8 0.4 2000 Wavenumber (cm-1) 3000 1000 Transmitance Transmitance
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter17: Applications Of Infrared Spectrometry
Section: Chapter Questions
Problem 17.4QAP
Related questions
Question
Propose one possible structure for each of the following based on the spectra provided. Use the Rule of 13 to find a formula for any questions that only provide the Molecular Weight. Also to perform an IHD calculations.Fully
interpret the IR spectra to justify the structure you have chosen.
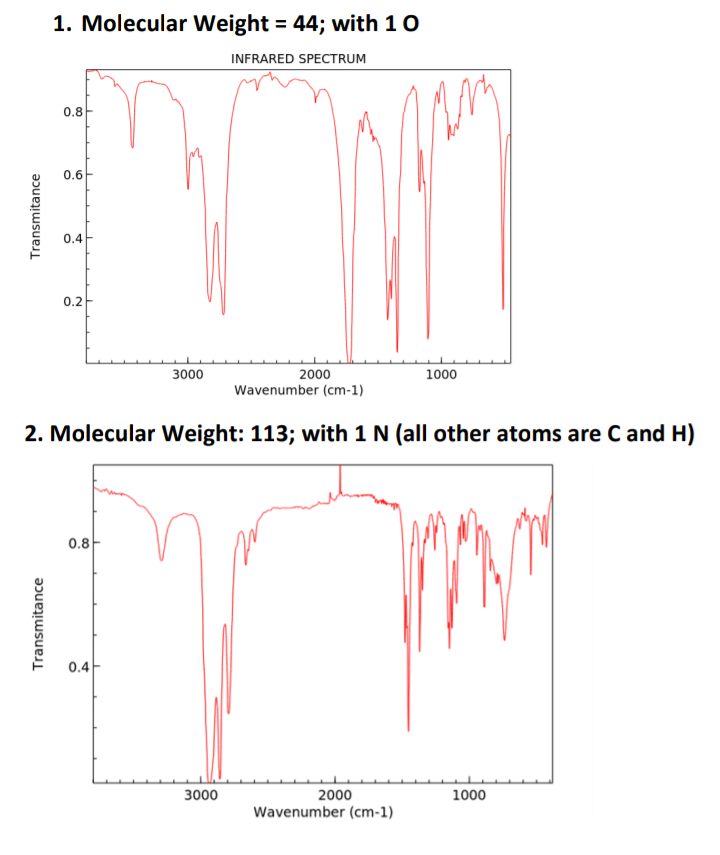
Transcribed Image Text:1. Molecular Weight = 44; with 10
INFRARED SPECTRUM
0.8
0.6
0.4
0.2
3000
2000
1000
Wavenumber (cm-1)
2. Molecular Weight: 113; with 1 N (all other atoms are C and H)
0.8
0.4
3000
1000
2000
Wavenumber (cm-1)
Transmitance
Transmitance
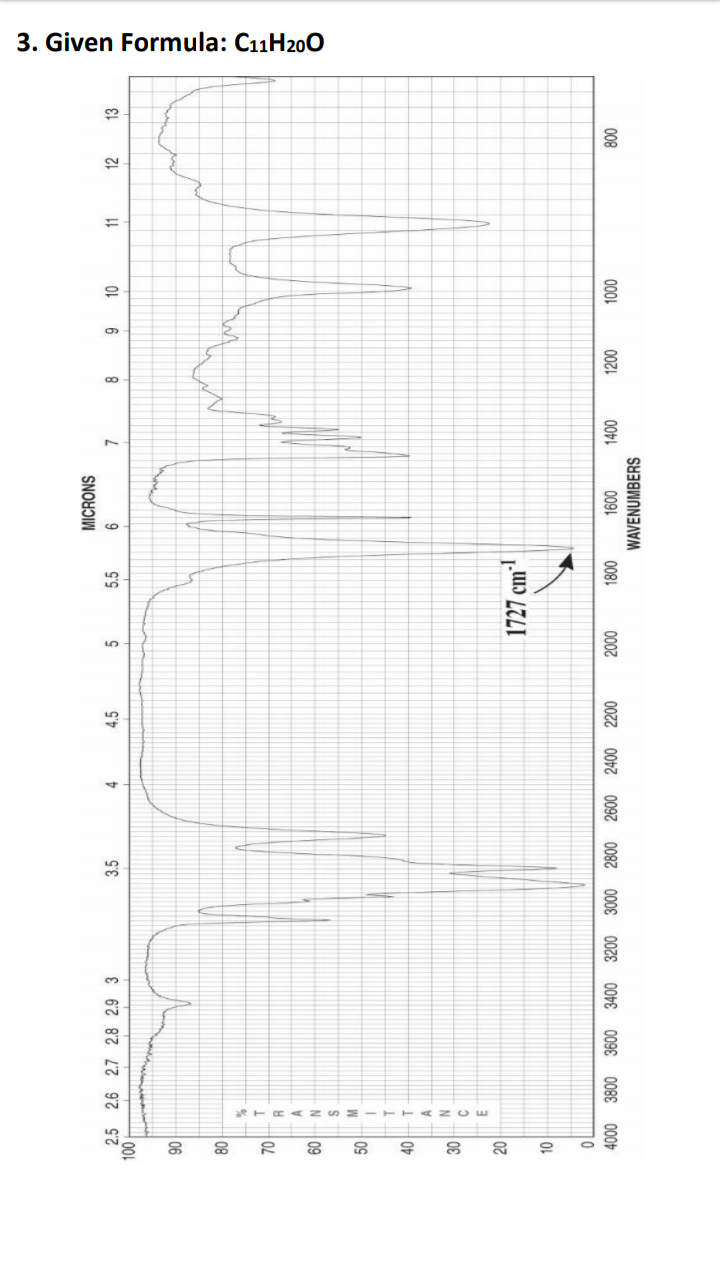
Transcribed Image Text:3. Given Formula: C11H200
70
50
40
30
008
WAVENUMBERS
0001
007
00+
009
0081
0007
2600 2400
0087
009E
3200 3000
008E
000
10
1727 cm³
N
09
08
06
13
9
00L
12
11
4.
5.5
4.5
SNOHJIW
3.5
2.5 2.6 2.7 2.8 2.9 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

