Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 38P: The percent ionic character of the bonds in several interhalogen Molecules (as estimated from their...
Related questions
Question
100%
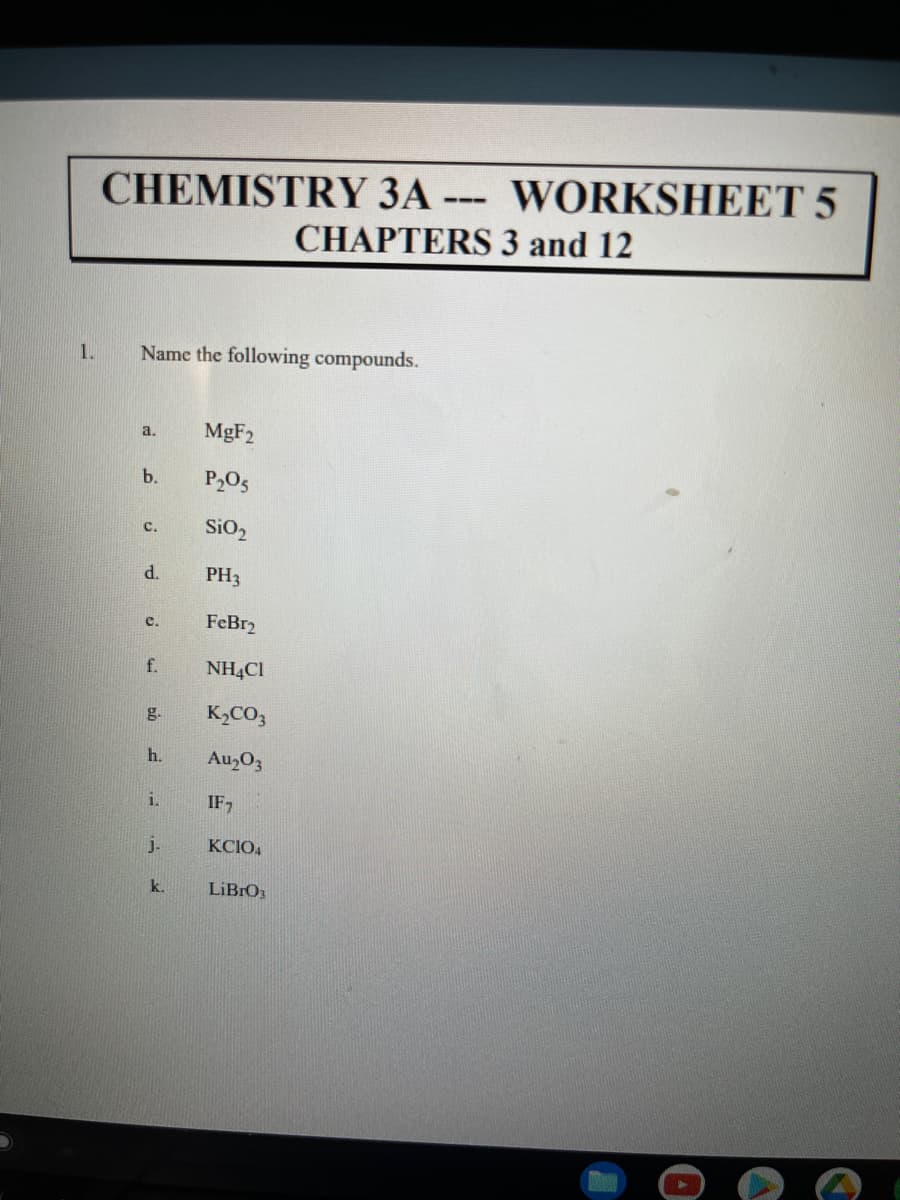
Transcribed Image Text:CHEMISTRY 3A --- WORKSHEET 5
CHAPTERS 3 and 12
1.
Name the following compounds.
MgF2
a.
b.
P205
SiO,
с.
d.
PH3
FeBr2
c.
f.
NH4CI
g.
K,CO3
h.
Au,O3
i.
IF,
j.
KCIO4
k.
LIBRO

Transcribed Image Text:2.
Write the formulas for the following compounds.
titanium(IV)oxide
a.
b.
aluminum bicarbonate
vanadium(IV)sulfide
C.
d.
magnesium nitrate
C.
dichlorine oxide
f.
magnesium sulfate
octasulfur
h.
nitrogen triiodide
i.
sodium phosphate
j-
sulfur hexafluoride
k.
iron(II) phosphite
calcium iodate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning