1. Over the years, the thermite reaction has been used for welding railroad rails, in incendiary bombs, and to ignite solid-fuel rocket motors. The reaction is: Fe2O3(e) + 2Als) → 2Fem + Al2O3(s) a. How many moles of Fe will be produced when there are 6 moles of Al ? b. What masses of iron(II) oxide and aluminum must be used to produce 15.0 g iron? DDT, an insecticide harmful to fish, birds, and humans, is produced by the following reaction: 2. 2C,H;CI + C,HOCI, C4H,Cls + H20 chlorobenzene chloral DDT In a government lab, 1142 g of chlorobenzene is reacted with 485 g of chloral. a. What mass of DDT is formed? b. Which reactant is limiting? Which is in excess? c. What mass of the excess reactant is left over? d. If the actual yield of DDT is 200.0 g, what is the percent yield?
1. Over the years, the thermite reaction has been used for welding railroad rails, in incendiary bombs, and to ignite solid-fuel rocket motors. The reaction is: Fe2O3(e) + 2Als) → 2Fem + Al2O3(s) a. How many moles of Fe will be produced when there are 6 moles of Al ? b. What masses of iron(II) oxide and aluminum must be used to produce 15.0 g iron? DDT, an insecticide harmful to fish, birds, and humans, is produced by the following reaction: 2. 2C,H;CI + C,HOCI, C4H,Cls + H20 chlorobenzene chloral DDT In a government lab, 1142 g of chlorobenzene is reacted with 485 g of chloral. a. What mass of DDT is formed? b. Which reactant is limiting? Which is in excess? c. What mass of the excess reactant is left over? d. If the actual yield of DDT is 200.0 g, what is the percent yield?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 117E: Bacterial digestion is an economical method of sewage treatment. The reaction is an intermediate...
Related questions
Question
Pls provide solution
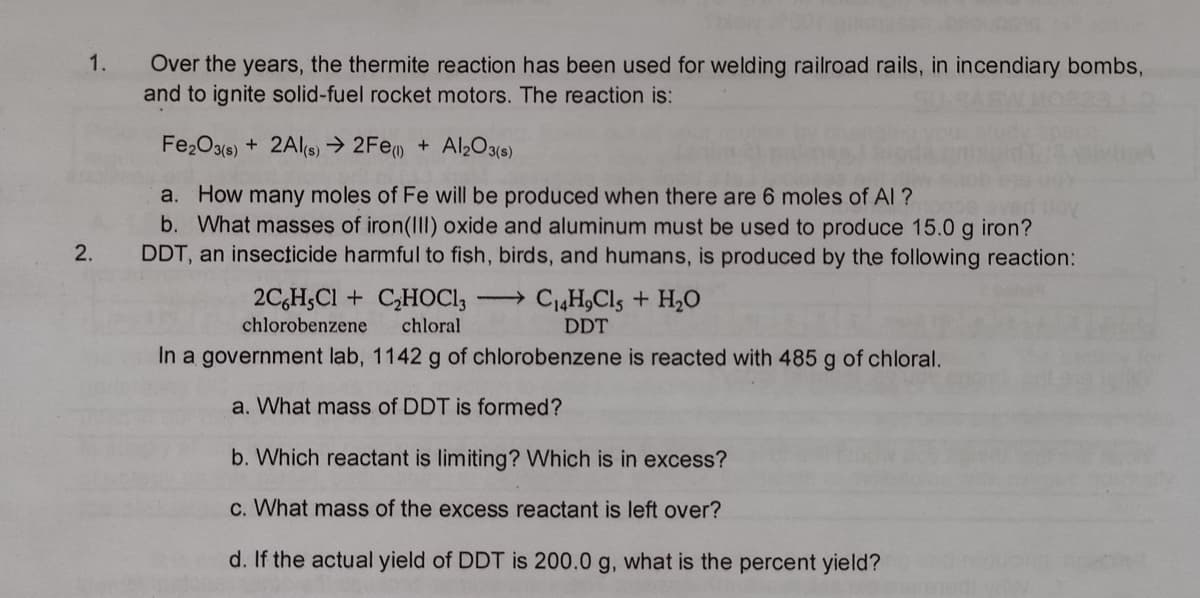
Transcribed Image Text:1.
Over the years, the thermite reaction has been used for welding railroad rails, in incendiary bombs,
and to ignite solid-fuel rocket motors. The reaction is:
Fe2O3(6) + 2Als) → 2Fem + Al2O3(s)
a. How many moles of Fe will be produced when there are 6 moles of Al ?
b. What masses of iron(II) oxide and aluminum must be used to produce 15.0 g iron?
DDT, an insecticide harmful to fish, birds, and humans, is produced by the following reaction:
2.
2C,H;Cl + C,HOC1,
C4H,Cls + H2O
chlorobenzene
chloral
DDT
In a government lab, 1142 g of chlorobenzene is reacted with 485 g of chloral.
a. What mass of DDT is formed?
b. Which reactant is limiting? Which is in excess?
c. What mass of the excess reactant is left over?
d. If the actual yield of DDT is 200.0 g, what is the percent yield?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning