m 2: magine you are working on ways to improve the process by which iron ore containing s converted into iron: Fe,03 (s) + 3 CO(g) → 2 Fe (s) + 3C02 (9) a) If you start with 150 g Fe2O3 as the limiting reactant, what is the theoretical yield of Fe? b) If your actual yield is 87.9 g, what is the percent yield?
m 2: magine you are working on ways to improve the process by which iron ore containing s converted into iron: Fe,03 (s) + 3 CO(g) → 2 Fe (s) + 3C02 (9) a) If you start with 150 g Fe2O3 as the limiting reactant, what is the theoretical yield of Fe? b) If your actual yield is 87.9 g, what is the percent yield?
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.65P: 4-65 Suppose the preparation of aspirin from salicylic acid and acetic anhydride (Problem 4-64)...
Related questions
Question
Answer problem No.2
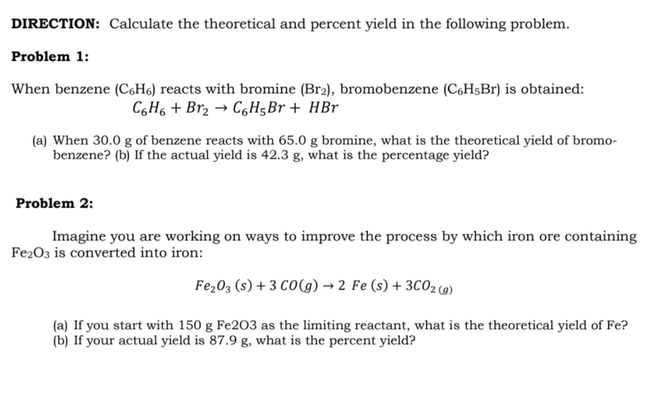
Transcribed Image Text:DIRECTION: Calculate the theoretical and percent yield in the following problem.
Problem 1:
When benzene (C,H6) reacts with bromine (Br2), bromobenzene (C6H$B1) is obtained:
C,H6 + Br2 → CgH;Br + HBr
(a) When 30.0 g of benzene reacts with 65.0 g bromine, what is the theoretical yield of bromo-
benzene? (b) If the actual yield is 42.3 g, what is the percentage yield?
Problem 2:
Imagine you are working on ways to improve the process by which iron ore containing
Fe203 is converted into iron:
Fe,03 (s) + 3 CO(g) → 2 Fe (s) + 3C02 (9)
(a) If you start with 150 g Fe2O3 as the limiting reactant, what is the theoretical yield of Fe?
(b) If your actual yield is 87.9 g, what is the percent yield?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning