INTERPRETING PHASE DIAGRAMS OF WATER AND CARBON DIOXIDE 218 Liquid B Liquid Solid Solid 5.2 Gas Gas 0.006 273 273.2 373 647 197.5 216.6 304.25 Phase diagram of water Phase diagram of CO, Based from the phase diagrams of water and carbon dioxide, answer the following questions and justify your answers: 1. You have ice at 263 K (-10.0°C) and 1.0 atm. What could you do to make the ice sublime? 2. A sample of dry ice (solid CO;) is cooled to 173 K (-100.0°C), and is set on a table at room temperature (298 K; 25°C). At what temperature is the rate of sublimation and deposition the same (assume that pressure is held constant at 1 atm)? (une) aunssaid Pressure (atm)
INTERPRETING PHASE DIAGRAMS OF WATER AND CARBON DIOXIDE 218 Liquid B Liquid Solid Solid 5.2 Gas Gas 0.006 273 273.2 373 647 197.5 216.6 304.25 Phase diagram of water Phase diagram of CO, Based from the phase diagrams of water and carbon dioxide, answer the following questions and justify your answers: 1. You have ice at 263 K (-10.0°C) and 1.0 atm. What could you do to make the ice sublime? 2. A sample of dry ice (solid CO;) is cooled to 173 K (-100.0°C), and is set on a table at room temperature (298 K; 25°C). At what temperature is the rate of sublimation and deposition the same (assume that pressure is held constant at 1 atm)? (une) aunssaid Pressure (atm)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter10: Solids, Liquids, And Phase Transitions
Section: Chapter Questions
Problem 69AP
Related questions
Question
Please help me answer these 2 questions!
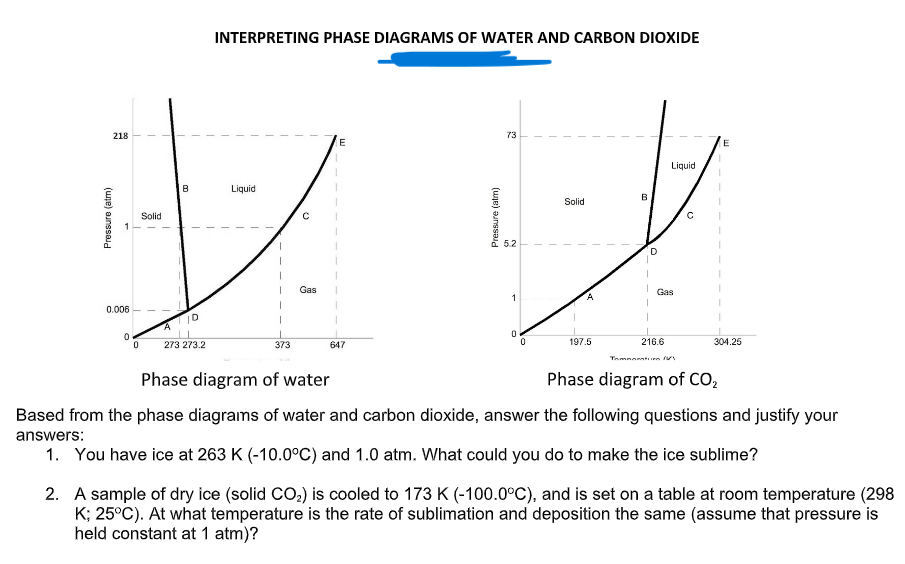
Transcribed Image Text:INTERPRETING PHASE DIAGRAMS OF WATER AND CARBON DIOXIDE
218
73
E
E
Liquid
B
Liquid
B
Solid
Solid
5.2
Gas
Gas
A.
0.006
273 273.2
373
647
197.5
216.6
304.25
Tnmnaraturn
Phase diagram of water
Phase diagram of CO,
Based from the phase diagrams of water and carbon dioxide, answer the following questions and justify your
answers:
1. You have ice at 263 K (-10.0°C) and 1.0 atm. What could you do to make the ice sublime?
2. A sample of dry ice (solid CO2) is cooled to 173 K (-100.0°C), and is set on a table at room temperature (298
K; 25°C). At what temperature is the rate of sublimation and deposition the same (assume that pressure is
held constant at 1 atm)?
(ue) aunssald
Pressure (atm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning