Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 52E: Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 6.00...
Related questions
Question
Transcribed Image Text:Copy of Spe
ent/d/1x04x_GDcrqdfD6R-r8-Pxn3rXs2vicUh-H7MN4NWds0/edit?pli.
100%
Normal text
Arlal Narrow
1.
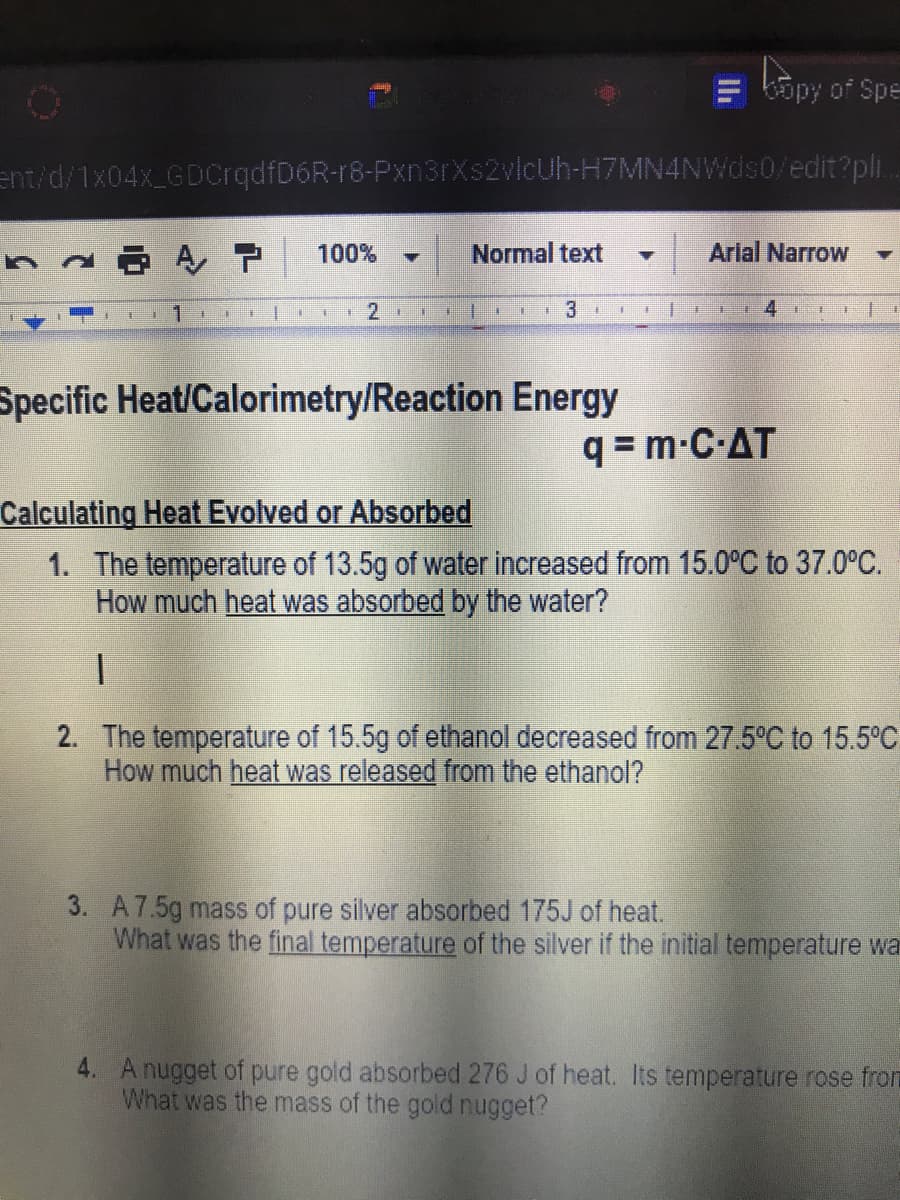
Specific Heat/Calorimetry/Reaction Energy
q = m-C-AT
Calculating Heat Evolved or Absorbed
1. The temperature of 13.5g of water increased from 15.0°C to 37.0°C.
How much heat was absorbed by the water?
2. The temperature of 15.5g of ethanol decreased from 27.5°C to 15.5°C
How much heat was released from the ethanol?
3. A7.5g mass of pure silver absorbed 175J of heat.
What was the final temperature of the silver if the initial temperature wa
4. A nugget of pure gold absorbed 276 J of heat. Its temperature rose from
What was the mass of the gold nugget?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax