1. To experiment with gas pressure and the ideal gas law, a student uses an open- ended manometer with a 7.2 L container for holding gas. The student then adds G.H g of methane gas (CH4) to the container at 10 °C. If atmospheric pressure is 107 kPa, (G and H are the last 2 digits of you ID number) A. Calculate how high the mercury will rise (mm) and in which direction (towards the gas or atmosphere), and draw a picture of the manometer with the CH4 gas inside. Assume ideal conditions. B. Describe two things you could do to the gas to make the liquid mercury levels equal (Ah = 0)? For each answer give an atomic-scale explanation of how that method will even out the mercury levels. 2. An unknown gas is found to have a density of G.H g/L. When the temperature is 79.1 °C, the pressure of the gas is 1.45 atm. What is the molar mass of the gas?
1. To experiment with gas pressure and the ideal gas law, a student uses an open- ended manometer with a 7.2 L container for holding gas. The student then adds G.H g of methane gas (CH4) to the container at 10 °C. If atmospheric pressure is 107 kPa, (G and H are the last 2 digits of you ID number) A. Calculate how high the mercury will rise (mm) and in which direction (towards the gas or atmosphere), and draw a picture of the manometer with the CH4 gas inside. Assume ideal conditions. B. Describe two things you could do to the gas to make the liquid mercury levels equal (Ah = 0)? For each answer give an atomic-scale explanation of how that method will even out the mercury levels. 2. An unknown gas is found to have a density of G.H g/L. When the temperature is 79.1 °C, the pressure of the gas is 1.45 atm. What is the molar mass of the gas?
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.114P: 5-114 Carbon dioxide gas, saturated with water vapor, can be produced by the addition of aqueous...
Related questions
Question
100%
G.H= 7.2< please help this work..
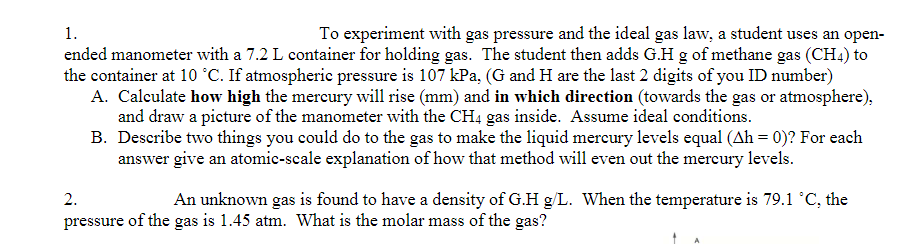
Transcribed Image Text:1.
To experiment with gas pressure and the ideal gas law, a student uses an open-
ended manometer with a 7.2 L container for holding gas. The student then adds G.H g of methane gas (CH4) to
the container at 10 °C. If atmospheric pressure is 107 kPa, (G and H are the last 2 digits of you ID number)
A. Calculate how high the mercury will rise (mm) and in which direction (towards the gas or atmosphere),
and draw a picture of the manometer with the CH4 gas inside. Assume ideal conditions.
B. Describe two things you could do to the gas to make the liquid mereury levels equal (Ah = 0)? For each
answer give an atomic-scale explanation of how that method will even out the mercury levels.
2.
An unknown gas is found to have a density of G.H g/L. When the temperature is 79.1 °C, the
pressure of the gas is 1.45 atm. What is the molar mass of the gas?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning