1. Use the melting point data to classify the ten substances into categories. List the categories, include the criteria for belonging to each group, and then place the substances into the appropriate groups. All data must be classified. Do NOT include the unknown substances (T, Q, H, Z, R)
1. Use the melting point data to classify the ten substances into categories. List the categories, include the criteria for belonging to each group, and then place the substances into the appropriate groups. All data must be classified. Do NOT include the unknown substances (T, Q, H, Z, R)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter21: Structure And Bonding In Solids
Section: Chapter Questions
Problem 36P
Related questions
Question
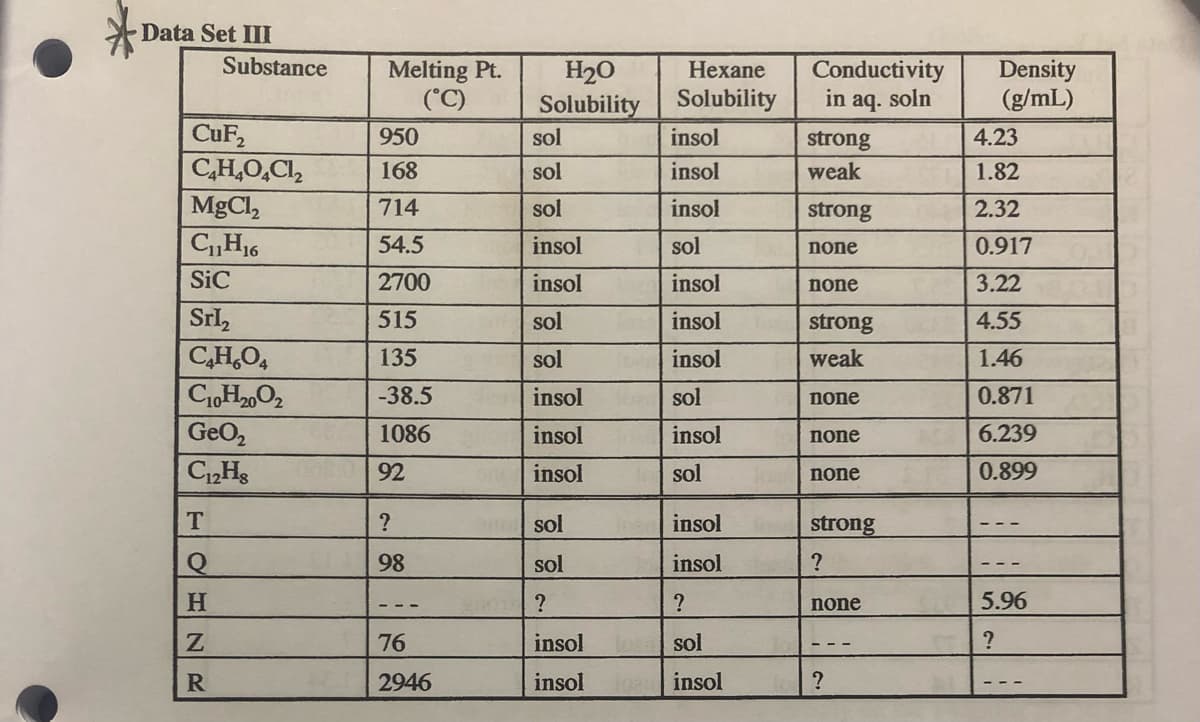
Transcribed Image Text:Data Set III
Substance
CuF₂
C₂H4O4Cl₂
| MgCl,
C₁1H₁6
SiC
Srl₂
C₂H₂O4
C10H₂0O2
GeO₂
C12HS
T
Q
H
Z
R
Melting Pt.
(°C)
950
168
714
54.5
2700
515
135
-38.5
1086
92
?
98
76
2946
H₂O
Solubility
sol
sol
sol
insol
insol
sol
sol
insol
insol
insol
sol
sol
?
insol
insol
Hexane
Solubility
insol
insol
insol
sol
insol
insol
insol
sol
insol
sol
insol
insol
?
sol
insol
Conductivity
in aq. soln
strong
weak
strong
none
none
strong
weak
none
none
none
strong
?
none
?
Density
(g/mL)
4.23
1.82
2.32
0.917
3.22
4.55
1.46
0.871
6.239
0.899
5.96
?
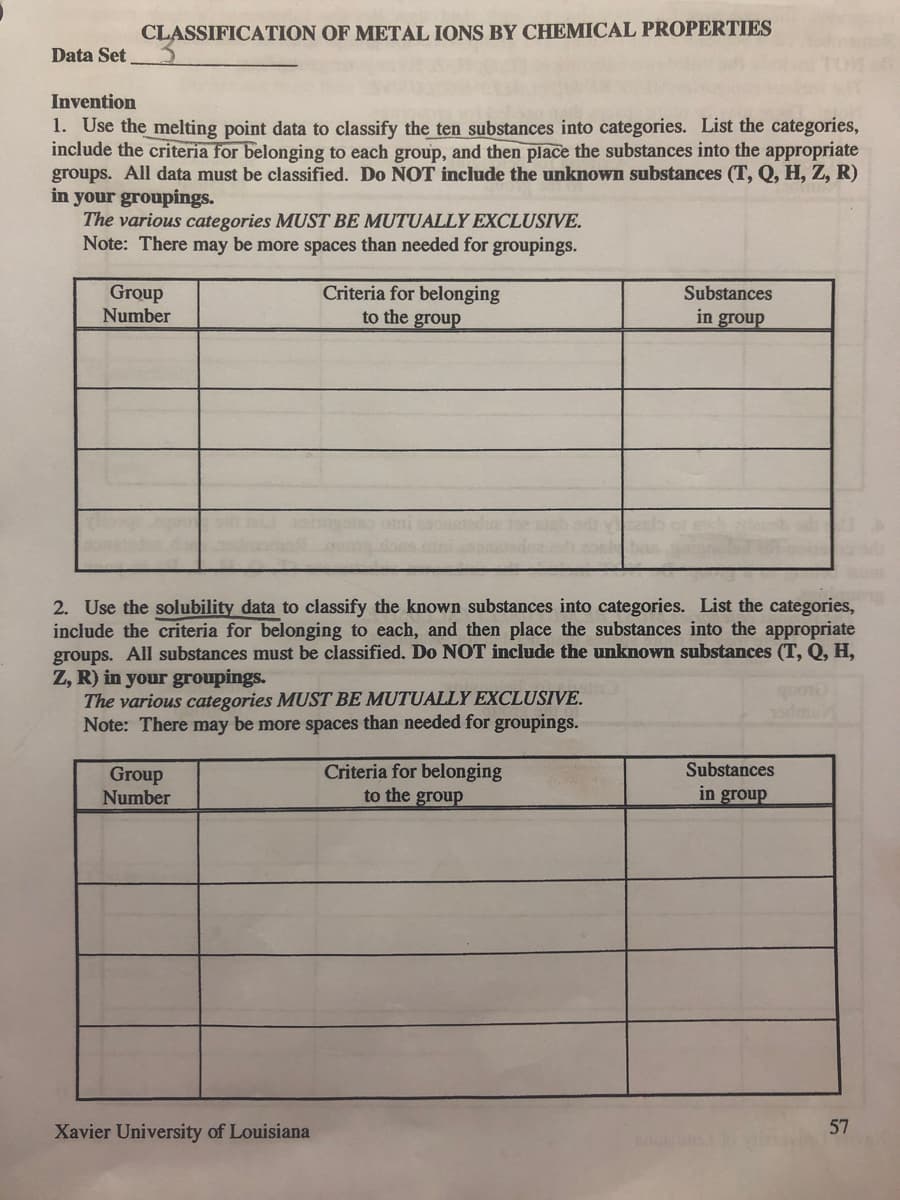
Transcribed Image Text:Data Set
CLASSIFICATION OF METAL IONS BY CHEMICAL PROPERTIES
Invention
1. Use the melting point data to classify the ten substances into categories. List the categories,
include the criteria for belonging to each group, and then place the substances into the appropriate
groups. All data must be classified. Do NOT include the unknown substances (T, Q, H, Z, R)
in your groupings.
The various categories MUST BE MUTUALLY EXCLUSIVE.
Note: There may be more spaces than needed for groupings.
Group
Number
Group
Number
Criteria for belonging
to the group
2. Use the solubility data to classify the known substances into categories. List the categories,
include the criteria for belonging to each, and then place the substances into the appropriate
groups. All substances must be classified. Do NOT include the unknown substances (T, Q, H,
Z, R) in your groupings.
The various categories MUST BE MUTUALLY EXCLUSIVE.
Note: There may be more spaces than needed for groupings.
Xavier University of Louisiana
Substances
in group
Criteria for belonging
to the group
Substances
in group
57
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning