Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 35E: Early tables of atomic weights (masses) were generated by measuring the mass of a substance that...
Related questions
Question
Please send me the question in 30 minutes it's very urgent plz
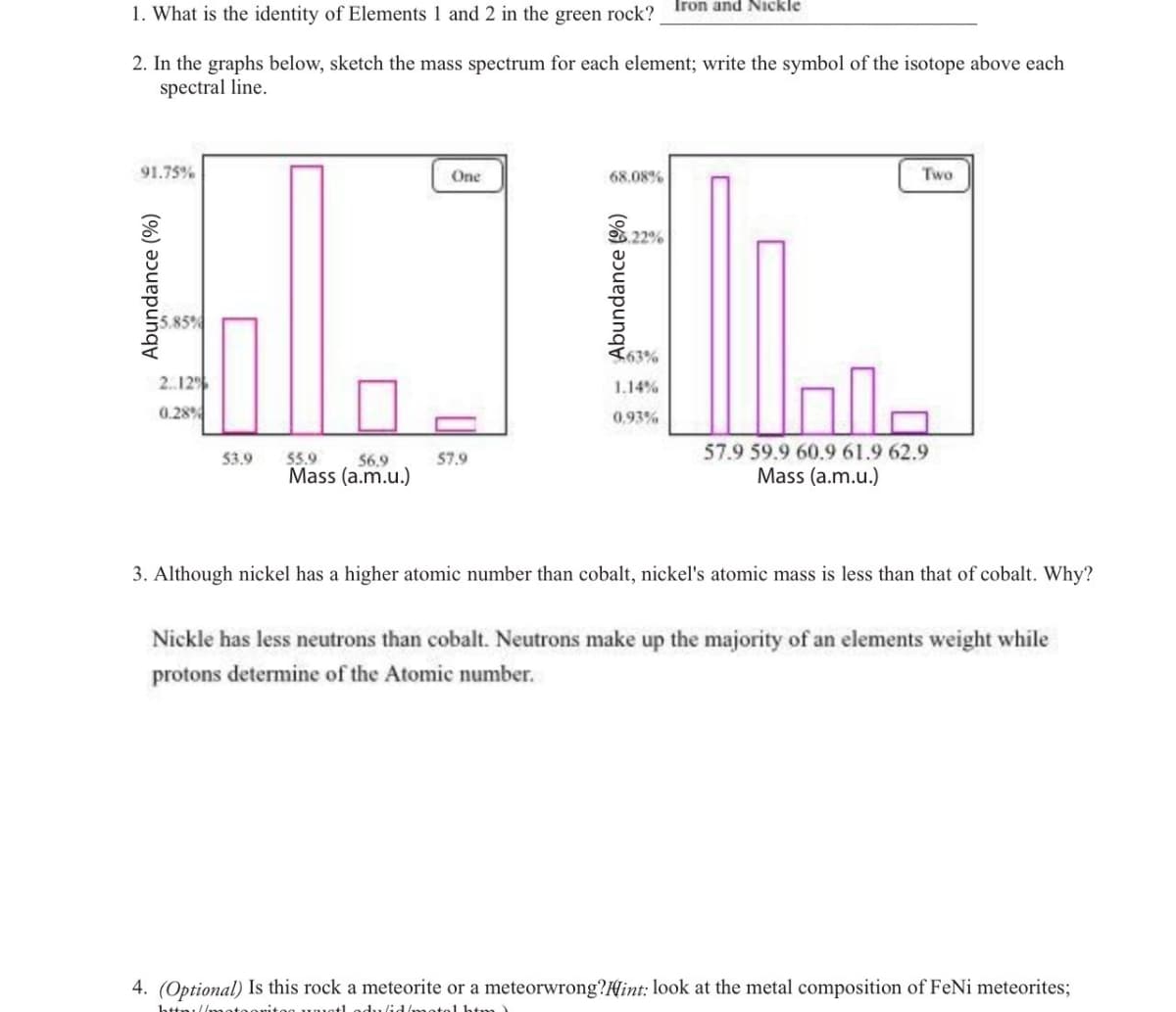
Transcribed Image Text:1. What is the identity of Elements 1 and 2 in the green rock? Iron and Nickle
2. In the graphs below, sketch the mass spectrum for each element; write the symbol of the isotope above each
spectral line.
91.75%
Abundance (%)
5.85%
2..12%
0.28%
53.9
56,9
55.9
Mass (a.m.u.)
One
57.9
68.08%
Abundance (%)
5.22%
463%
1.14%
0,93%
Two
57.9 59.9 60.9 61.9 62.9
Mass (a.m.u.)
3. Although nickel has a higher atomic number than cobalt, nickel's atomic mass is less than that of cobalt. Why?
Nickle has less neutrons than cobalt. Neutrons make up the majority of an elements weight while
protons determine of the Atomic number.
4. (Optional) Is this rock a meteorite or a meteorwrong?Hint: look at the metal composition of FeNi meteorites;
http://matoonitog umotl dulid/motal htm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning