Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 74QAP: Fifty cm3 of 1.000 M nitrous acid is titrated with 0.850 M NaOH. What is the pH of the solution (a)...
Related questions
Question
Transcribed Image Text:Paragraph
Styles
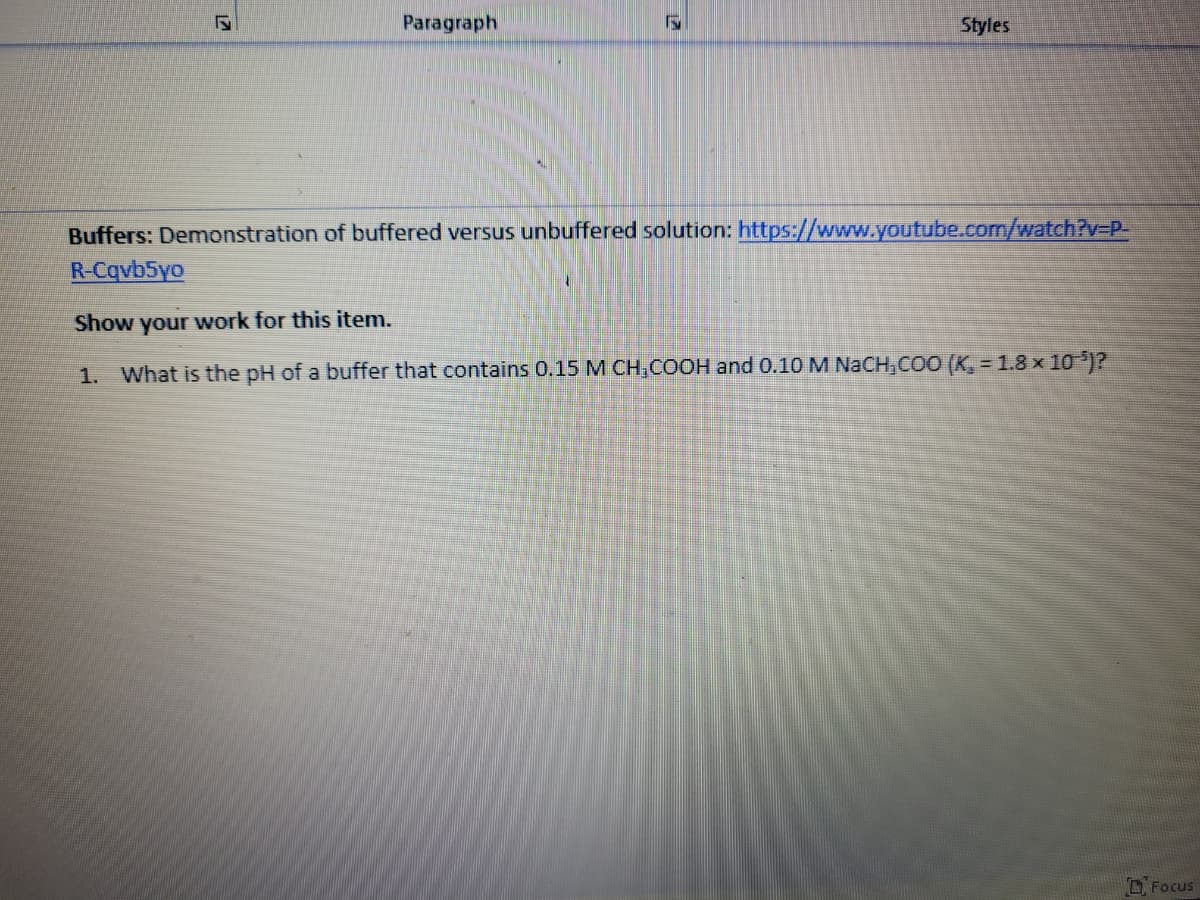
Buffers: Demonstration of buffered versus unbuffered solution: https://www.youtube.com/watch?v3DP-
R-Cqvb5yo
Show your work for this item.
1. What is the pH of a buffer that contains 0.15 M CH,COOH and 0.10 M NACH,COO (K, = 1.8 x 10 )?
Focus
Expert Solution
Step 1
1)
Given,
Concentration of CH3COOH solution = 0.15 M
[CH3COOH] = 0.15 M
Concentration of NaCH3COO solution = 0.10 M
[NaCH3COO] = 0.10 M
Acid dissociation constant (Ka) for CH3COOH = 1.8 × 10-5
pH of a buffer solution = ?
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning