A particular mass of nitrogen gas, N2, occupies a volume of 1.00 L at -50 °C and 800 bar. Given the compressibility factor, Z, values for N2 are 1.95 at -50 °C and 800 bar and 1.10 at 100 °C and 200 bar, respectively. (i) Determine the volume occupied by the same mass of N2 at 100 °C and 200 bar using the Z values for N2. (ii) Using the obtained value in 1(a)(i), calculate its error using the volume obtained ideal gas law value.
A particular mass of nitrogen gas, N2, occupies a volume of 1.00 L at -50 °C and 800 bar. Given the compressibility factor, Z, values for N2 are 1.95 at -50 °C and 800 bar and 1.10 at 100 °C and 200 bar, respectively. (i) Determine the volume occupied by the same mass of N2 at 100 °C and 200 bar using the Z values for N2. (ii) Using the obtained value in 1(a)(i), calculate its error using the volume obtained ideal gas law value.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.103QP: Calculate the molar volume of ethane at 1.00 atm and 0C and at 10.0 atm and 0C, using the van der...
Related questions
Question
I put the answer for 1(a)(i), need the answer for 1(a)(ii).

Transcribed Image Text:I@)0) eV
nR=
comptont
IT
Pf xVE
I4 x Tt
li x Vi
Li x T;
Pi
intal pressue- foo bar
Vi = INhal rolume
Li = 1.95
1.00L
=INhal tem peatre
hnal pressue
Ti
273+(-50) - 223 K
Pe
fimal Bolune
200ban
VF
If
Temperatue final = 273+100
1.10
%3D
T年 =
= 373 K
s00 X 1.0O
200 x Vf
1.95 メ233
1.10 x 373
Vtina
800 X 1.00
1.95 x 223
J.10x 373
メ
3.774L apprix.
200
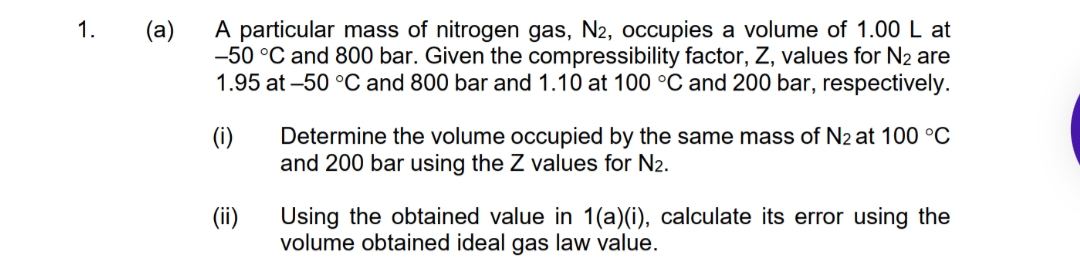
Transcribed Image Text:A particular mass of nitrogen gas, N2, occupies a volume of 1.00 L at
-50 °C and 800 bar. Given the compressibility factor, Z, values for N2 are
1.95 at -50 °C and 800 bar and 1.10 at 100 °C and 200 bar, respectively.
1.
(a)
(i)
Determine the volume occupied by the same mass of N2 at 100 °C
and 200 bar using the Z values for N2.
(ii)
Using the obtained value in 1(a)(i), calculate its error using the
volume obtained ideal gas law value.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax