1. What will be the primary consideration in solving for concentration of solutions? 2. What is the general formula in determining the % concentration regardless of the phase of solute and solvent? 3. How will you know when to use mass/mass %? Volume/volume %? Mass/volume %?
1. What will be the primary consideration in solving for concentration of solutions? 2. What is the general formula in determining the % concentration regardless of the phase of solute and solvent? 3. How will you know when to use mass/mass %? Volume/volume %? Mass/volume %?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 6QAP
Related questions
Question

Transcribed Image Text:Answer the following questions.
1. What will be the primary consideration in solving for concentration of
solutions?
2. What is the general formula in determining the % concentration regardless of
the phase of solute and solvent?
3. How will you know when to use mass/mass %? Volume/volume %?
Mass/volume %?
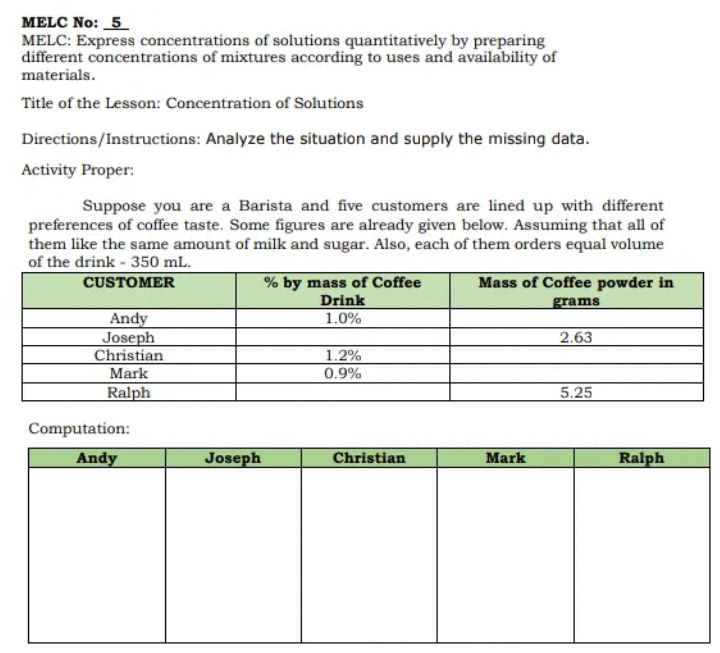
Transcribed Image Text:MELC No: _5
MELC: Express concentrations of solutions quantitatively by preparing
different concentrations of mixtures according to uses and availability of
materials.
Title of the Lesson: Concentration of Solutions
Directions/Instructions: Analyze the situation and supply the missing data.
Activity Proper:
Suppose you are a Barista and five customers are lined up with different
preferences of coffee taste. Some figures are already given below. Assuming that all of
them like the same amount of milk and sugar. Also, each of them orders equal volume
of the drink - 350 mL.
CUSTOMER
Mass of Coffee powder in
% by mass of Coffee
Drink
1.0%
grams
Andy
Joseph
Christian
2.63
1.2%
0.9%
Mark
Ralph
5.25
Computation:
Andy
Joseph
Christian
Mark
Ralph
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning