1. Which nitrogen-containing compound of each pair would you expect to be stronger base? Indicate (a) or (b) on the box provided. i. (а) (b) (ii) O,N- -NH2 or O,N- -NH, i. (a) H3C CH3 (b) H;C CH3 (ii) H;C, CH3 or 2. Which carboxylic compound of each pair would you expect to be stronger acid? Indicate (a) or (b) on the box provided. i. (a) (b) (i) OH or Me,Ň ii. (a) (Б) (ii) OH OH or CF3
1. Which nitrogen-containing compound of each pair would you expect to be stronger base? Indicate (a) or (b) on the box provided. i. (а) (b) (ii) O,N- -NH2 or O,N- -NH, i. (a) H3C CH3 (b) H;C CH3 (ii) H;C, CH3 or 2. Which carboxylic compound of each pair would you expect to be stronger acid? Indicate (a) or (b) on the box provided. i. (a) (b) (i) OH or Me,Ň ii. (a) (Б) (ii) OH OH or CF3
Chapter22: Carbonyl Alpha-substitution Reactions
Section22.SE: Something Extra
Problem 18VC
Related questions
Question
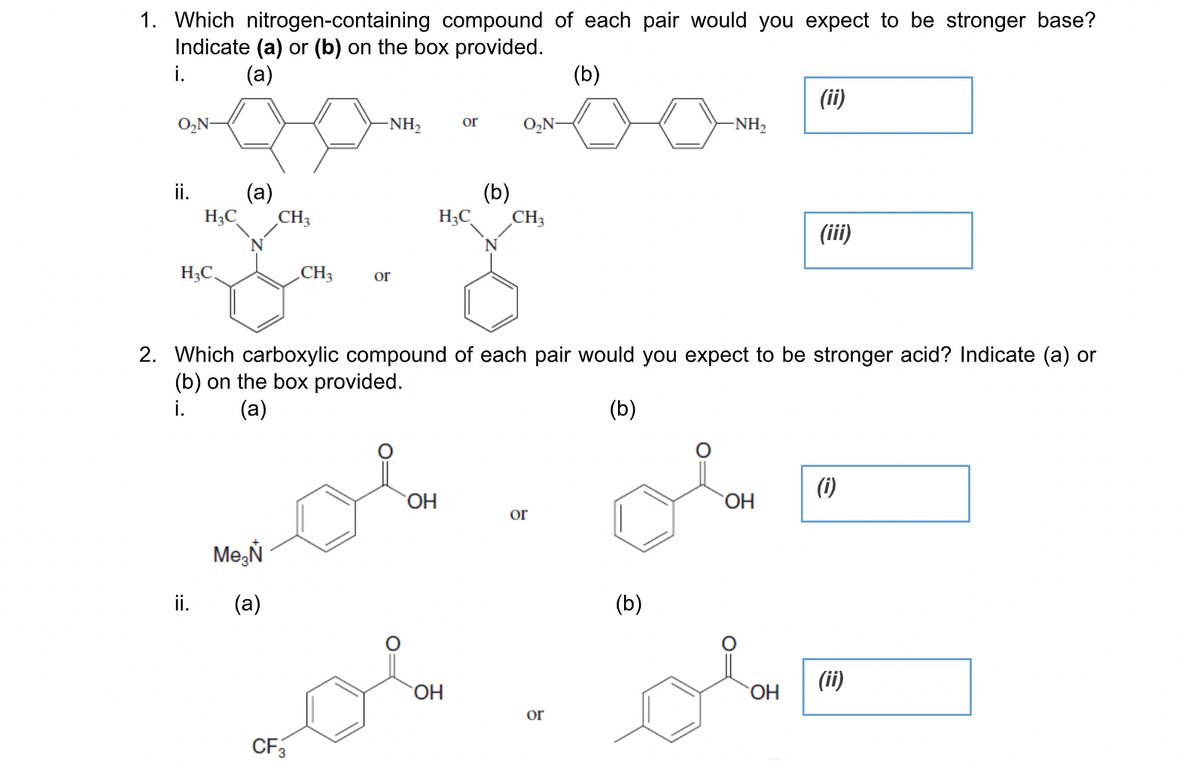
Transcribed Image Text:1. Which nitrogen-containing compound of each pair would you expect to be stronger base?
Indicate (a) or (b) on the box provided.
i.
(a)
(b)
(ii)
O,N-
NH,
or
-NH2
ii.
(a)
(b)
H3C
CH3
H;C
CH3
(iii)
N
H;C.
CH3
or
2. Which carboxylic compound of each pair would you expect to be stronger acid? Indicate (a) or
(b) on the box provided.
i.
(a)
(b)
(i)
OH
ОН
or
Me,Ň
ii.
(a)
(b)
(ii)
HO.
ОН
or
CF3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

