1. You measured out 1.4315g of oxalic acid dihydrate and dissolved it into a 100.00mL volumetric flask. Calculate the concentration of the oxalic acid.
1. You measured out 1.4315g of oxalic acid dihydrate and dissolved it into a 100.00mL volumetric flask. Calculate the concentration of the oxalic acid.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 118CP: Malonic acid (HO2CCH2CO2H) is a diprotic acid. In the titration of malonic acid w ith NaOH,...
Related questions
Question
Please answer Q1
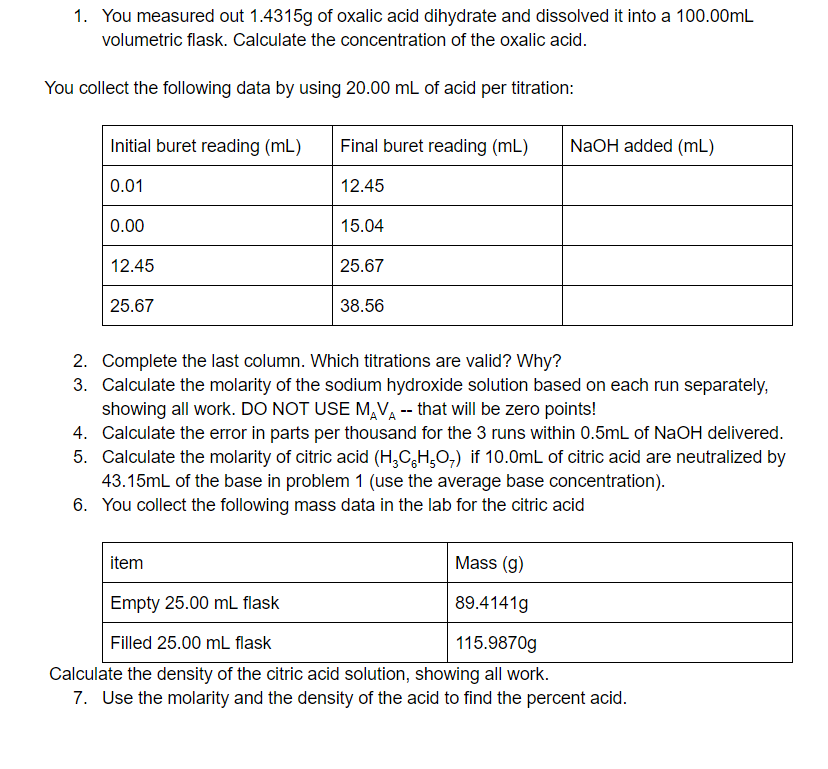
Transcribed Image Text:1. You measured out 1.4315g of oxalic acid dihydrate and dissolved it into a 100.00mL
volumetric flask. Calculate the concentration of the oxalic acid.
You collect the following data by using 20.00 mL of acid per titration:
Initial buret reading (mL)
Final buret reading (mL)
NaOH added (mL)
0.01
12.45
0.00
15.04
12.45
25.67
25.67
38.56
2. Complete the last column. Which titrations are valid? Why?
3. Calculate the molarity of the sodium hydroxide solution based on each run separately,
showing all work. DO NOT USE M,VA-- that will be zero points!
4. Calculate the error in parts per thousand for the 3 runs within 0.5mL of NaOH delivered.
5. Calculate the molarity of citric acid (H,C,H,O,) if 10.0mL of citric acid are neutralized by
43.15ml of the base in problem 1 (use the average base concentration).
6. You collect the following mass data in the lab for the citric acid
item
Mass (g)
Empty 25.00 mL flask
89.4141g
Filled 25.00 mL flask
115.9870g
Calculate the density of the citric acid solution, showing all work.
7. Use the molarity and the density of the acid to find the percent acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning