1.2 In the food industry, food safety is a major concern. For example, the food-dye, FD&C Yellow 6, is approved for use in foods but the acceptable daily intake by children is 3.75 mg/kg due to concerns like hyperactivity. A UV-vis spectroscopy calibration curve for yellow dye 6 (absorbance vs. concentration in mol/L) was prepared at 485nm. The equation of the linear region between 0.2 and 1.4 absorbance is: y = 19896x Determine the molar extinction coefficient of yellow dye 6 at 485 nm. a. Assume the pathlength is 1.000 cm.
1.2 In the food industry, food safety is a major concern. For example, the food-dye, FD&C Yellow 6, is approved for use in foods but the acceptable daily intake by children is 3.75 mg/kg due to concerns like hyperactivity. A UV-vis spectroscopy calibration curve for yellow dye 6 (absorbance vs. concentration in mol/L) was prepared at 485nm. The equation of the linear region between 0.2 and 1.4 absorbance is: y = 19896x Determine the molar extinction coefficient of yellow dye 6 at 485 nm. a. Assume the pathlength is 1.000 cm.
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 4P
Related questions
Question
PLEASE ANSWER ASAP

Transcribed Image Text:1.2 In the food industry, food safety is a major concern. For example, the food-dye,
FD&C Yellow 6, is approved for use in foods but the acceptable daily intake by children
is 3.75 mg/kg due to concerns like hyperactivity.
A UV-vis spectroscopy calibration curve for yellow dye 6 (absorbance vs. concentration
in mol/L) was prepared at 485nm. The equation of the linear region between 0.2 and 1.4
absorbance is:
y = 19896x
Determine the molar extinction coefficient of yellow dye 6 at 485 nm.
a.
Assume the pathlength is 1.000 cm.
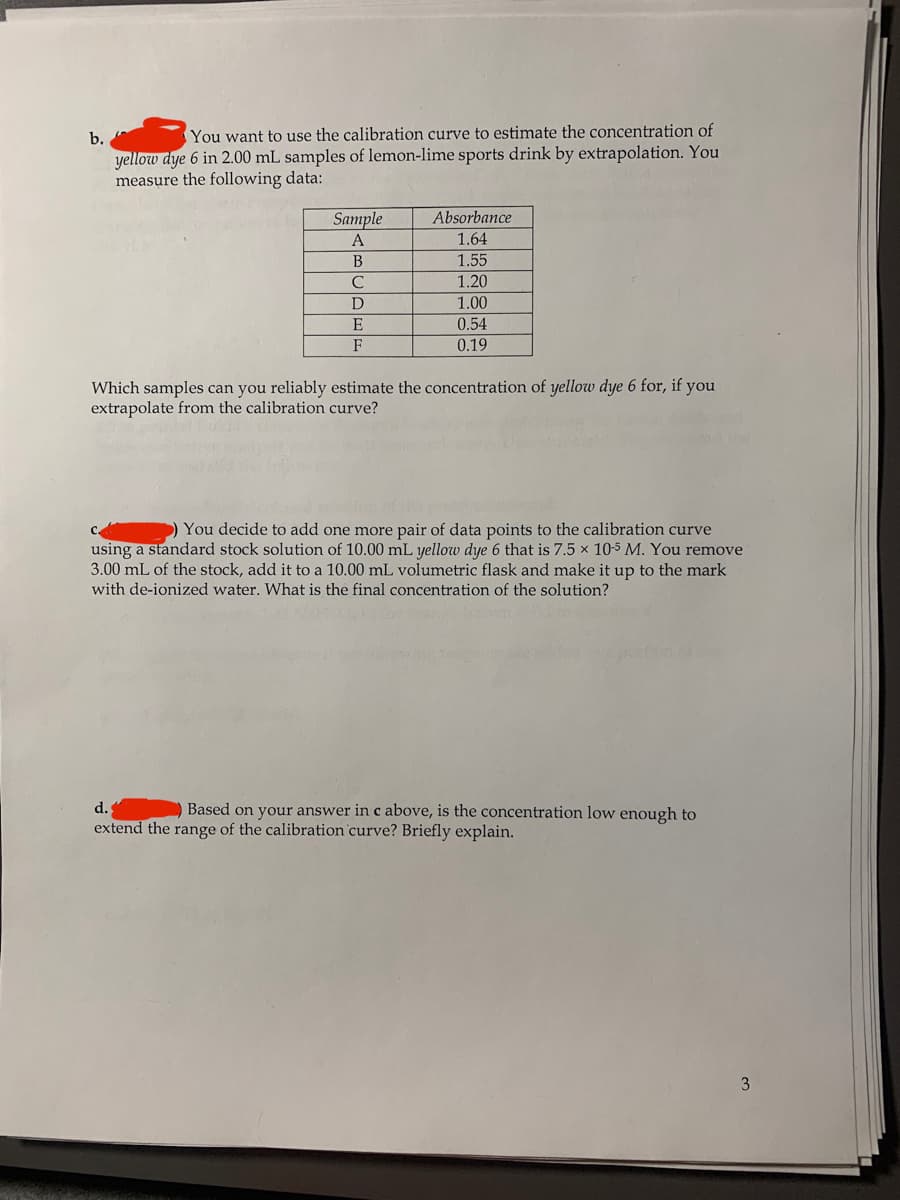
Transcribed Image Text:b.
You want to use the calibration curve to estimate the concentration of
yellow dye 6 in 2.00 mL samples of lemon-lime sports drink by extrapolation. You
measure the following data:
Sample
Absorbance
1.64
1.55
A
B
1.20
D
1.00
E
0.54
F
0.19
Which samples can you reliably estimate the concentration of yellow dye 6 for, if you
extrapolate from the calibration curve?
) You decide to add one more pair of data points to the calibration curve
using a standard stock solution of 10.00 mL yellow dye 6 that is 7.5 x 10-5 M. You remove
3.00 mL of the stock, add it to a 10.00 mL volumetric flask and make it up to the mark
with de-ionized water. What is the final concentration of the solution?
d.
Based on your answer in c above, is the concentration low enough to
extend the range of the calibration curve? Briefly explain.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
