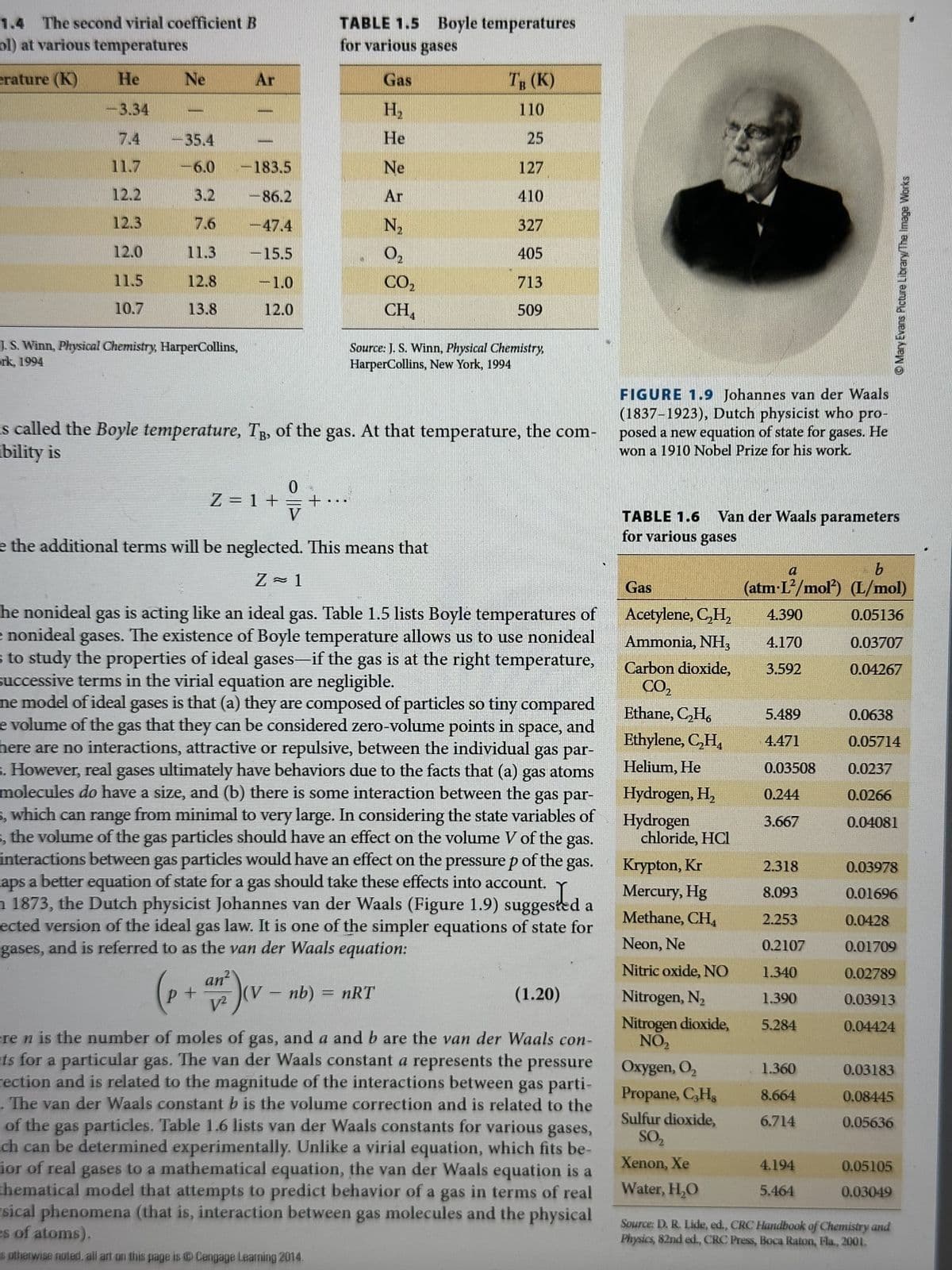
1.38. Calculate the Boyle temperatures for carbon dioxide, oxygen, and nitrogen using the van der Waals constants in Table 1.6. How close do they come to the experimental values from Table 1.5?
1.38. Calculate the Boyle temperatures for carbon dioxide, oxygen, and nitrogen using the van der Waals constants in Table 1.6. How close do they come to the experimental values from Table 1.5?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.38E: Calculate the Boyle temperatures for carbon dioxide, oxygen, and nitrogen using the van der Waals...
Related questions
Question
Help me with explanations and show me how to solve I feel lost

Transcribed Image Text:1.38. Calculate the Boyle temperatures for carbon dioxide,
oxygen, and nitrogen using the van der Waals constants in
Table 1.6. How close do they come to the experimental values
from Table 1.5?
Transcribed Image Text:1.4 The second virial coefficient B
ol) at various temperatures
erature (K)
He
-3.34
7.4
11.7
12.2
12.3
12.0
11.5
10.7
Ne
Ar
-35.4
-6.0 -183.5
3.2
-86.2
7.6
-47.4
11.3
-15.5
12.8
-1.0
13.8
12.0
J. S. Winn, Physical Chemistry, HarperCollins,
ork, 1994
Z=1+
0
TABLE 1.5 Boyle temperatures
for various gases
+
is called the Boyle temperature, TB, of the gas. At that temperature, the com-
ibility is
Gas
H₂
He
Ne
Ar
N₂
0₂
CO₂
CH₁
Source: J. S. Winn, Physical Chemistry,
HarperCollins, New York, 1994
TB (K)
110
25
127
410
327
405
713
509
e the additional terms will be neglected. This means that
Z=1
nRT
is
acting like an
he nonideal gas is acting like an ideal
ideal gas. Table 1.5 lists Boyle temperatures of
nonideal gases. The existence of Boyle temperature allows us to use nonideal
s to study the properties of ideal gases-if the gas is at the right temperature,
successive terms in the virial equation are negligible.
ne model of ideal gases is that (a) they are composed of particles so tiny compared
e volume of the gas that they can be considered zero-volume points in space, and
here are no interactions, attractive or repulsive, between the individual gas par-
However, real gases ultimately have behaviors due to the facts that (a) gas atoms
molecules do have a size, and (b) there is some interaction between the gas par-
5, which can range from minimal to very large. In considering the state variables of
5, the volume of the gas particles should have an effect on the volume V of the gas.
interactions between gas particles would have an effect on the pressure p of the gas.
aps a better equation of state for a gas should take these effects into account.
1873, the Dutch physicist Johannes van der Waals (Figure 1.9) suggested
ected version of the ideal gas law. It is one of the simpler equations of state for
gases, and is referred to as the van der Waals equation:
an²
(V
(p+ an)(v - nb)
(1.20)
ere n is the number of moles of gas, and a and b are the van der Waals con-
ts for a particular gas. The van der Waals constant a represents the pressure
rection and is related to the magnitude of the interactions between gas parti-
The van der Waals constant b is the volume correction and is related to the
of the gas particles. Table 1.6 lists van der Waals constants for various gases,
ch can be determined experimentally. Unlike a virial equation, which fits be-
ior of real gases to a mathematical equation, the van der Waals equation is a
thematical model that attempts to predict behavior of a gas in terms of real
sical phenomena (that is, interaction between gas molecules and the physical
es of atoms).
s otherwise noted, all art on this page is Cengage Learning 2014.
FIGURE 1.9 Johannes van der Waals
(1837-1923), Dutch physicist who pro-
posed a new equation of state for gases. He
won a 1910 Nobel Prize for his work.
TABLE 1.6 Van der Waals parameters
for various gases
Gas
Acetylene, C₂H₂
Ammonia, NH3
Carbon dioxide,
CO₂
Ethane, C₂H6
Ethylene, C₂H₁
Helium, He
Hydrogen, H₂
Hydrogen
chloride, HCl
Krypton, Kr
Mercury, Hg
Methane, CH4
Neon, Ne
Nitric oxide, NO
Nitrogen, N₂
Nitrogen dioxide,
NO₂
Oxygen, O₂
Propane, C3H8
Sulfur dioxide,
SO₂
Xenon, Xe
Water, H₂O
a
b
(atm-L²/mol) (L/mol)
0.05136
4.390
4.170
3.592
5.489
4.471
0.03508
0.244
3.667
2.318
8.093
2.253
0.2107
1.340
1.390
5.284
1.360
8.664
6.714
4.194
5.464
0.03707
0.04267
ⒸMary Evans Picture Library/The Image Works
0.0638
0.05714
0.0237
0.0266
0.04081
0.03978
0.01696
0.0428
0.01709
0.02789
0.03913
0.04424
0.03183
0.08445
0.05636
0.05105
0.03049
Source: D. R. Lide, ed., CRC Handbook of Chemistry and
Physics, 82nd ed., CRC Press, Boca Raton, Fla., 2001.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning