1.51 g H2 is allowed to react with 10.0 g N2, producing 2.01 g NH3. Part A What is the theoretical yield in grams for this reaction under the given conditions? Part B What is the percent yield for this reaction under the given conditions?
1.51 g H2 is allowed to react with 10.0 g N2, producing 2.01 g NH3. Part A What is the theoretical yield in grams for this reaction under the given conditions? Part B What is the percent yield for this reaction under the given conditions?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 37P: The iron oxide Fe2O3 reacts with carbon monoxide (CO)to give iron and carbon dioxide:...
Related questions
Question
100%
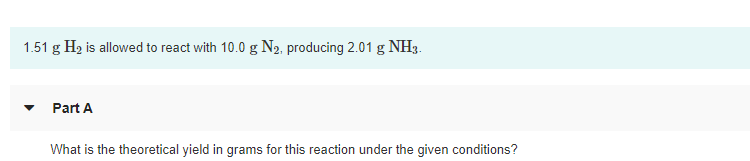
Transcribed Image Text:1.51 g H2 is allowed to react with 10.0 g N2, producing 2.01 g NH3.
Part A
What is the theoretical yield in grams for this reaction under the given conditions?
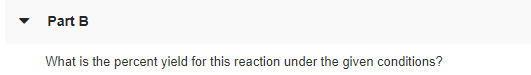
Transcribed Image Text:Part B
What is the percent yield for this reaction under the given conditions?
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps with 5 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning