Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter27: Amines
Section: Chapter Questions
Problem 5E
Related questions
Question
Please answer all of them
![5. What is the name of the product in the following reaction?
6.
TRUE
or
conc. KMnO4, heat
FALSE. The reactions shown below will produce an aldehyde.
2000 Jour
"CO,H
1. SOC1₂
2. LIAI[OC(CH3)3]3H](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fe1657792-05f2-4f41-a2f4-a113dcf6b483%2Fdc93f259-5143-4d78-a78a-b17785a71197%2Fo7ni2w_processed.jpeg&w=3840&q=75)
Transcribed Image Text:5. What is the name of the product in the following reaction?
6.
TRUE
or
conc. KMnO4, heat
FALSE. The reactions shown below will produce an aldehyde.
2000 Jour
"CO,H
1. SOC1₂
2. LIAI[OC(CH3)3]3H
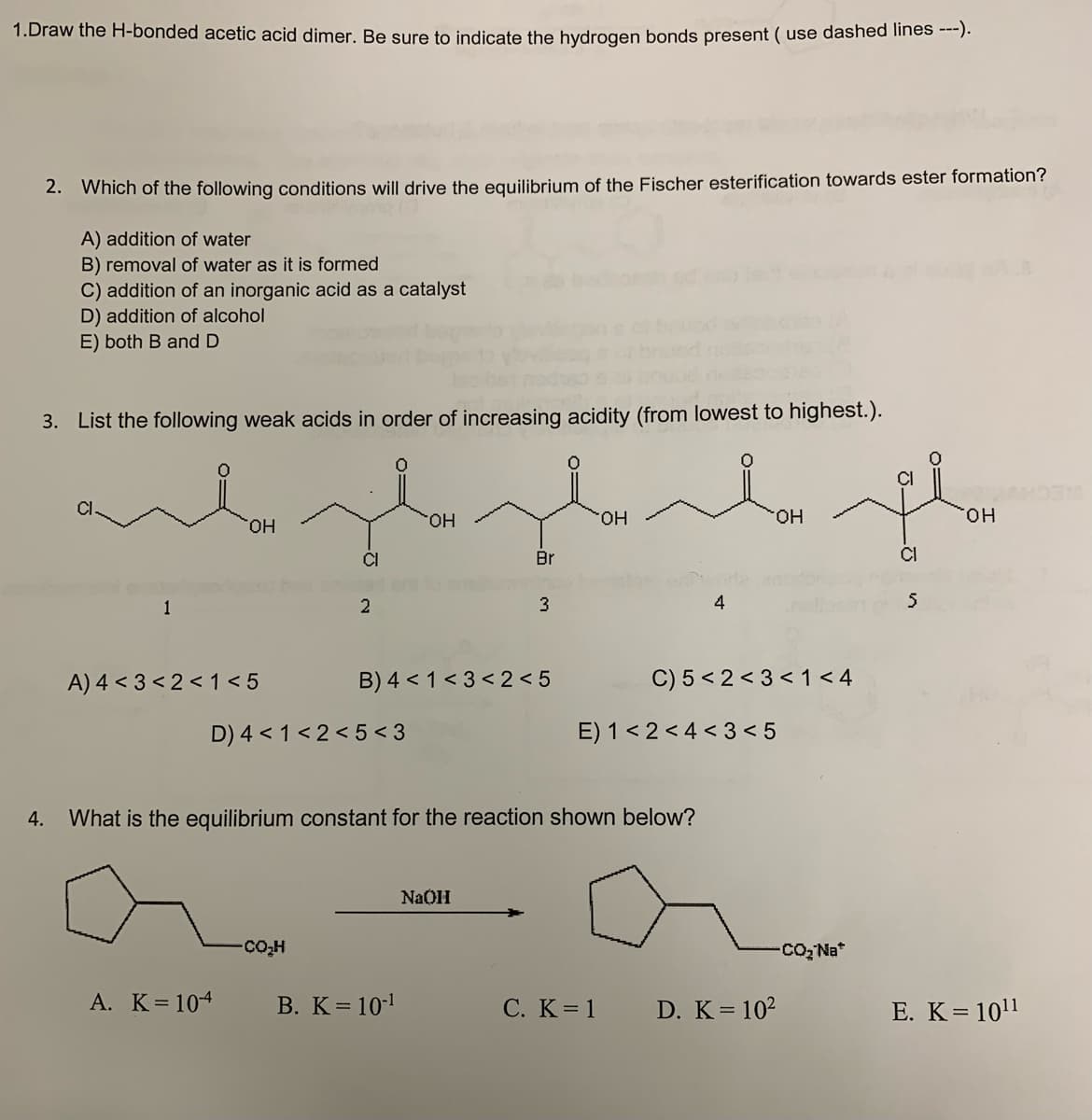
Transcribed Image Text:1.Draw the H-bonded acetic acid dimer. Be sure to indicate the hydrogen bonds present (use dashed lines ---).
2. Which of the following conditions will drive the equilibrium of the Fischer esterification towards ester formation?
A) addition of water
B) removal of water as it is formed
C) addition of an inorganic acid as a catalyst
D) addition of alcohol
E) both B and D
3. List the following weak acids in order of increasing acidity (from lowest to highest.).
1
OH
A) 4 <3 <2< 1<5
A. K = 104
CI
2
D) 4 < 1<2<5<3
COH
OH
B) 4 < 1<3 <2<5
B. K = 10-¹
Br
3
NaOH
CI
derde pla
OH
OH
CI
4. What is the equilibrium constant for the reaction shown below?
4
E) 1<2<4<3<5
C. K = 1
C) 5 <2<3 < 1<4
OH
D. K = 10²
-CO₂ Na*
5
E. K = 10¹1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning