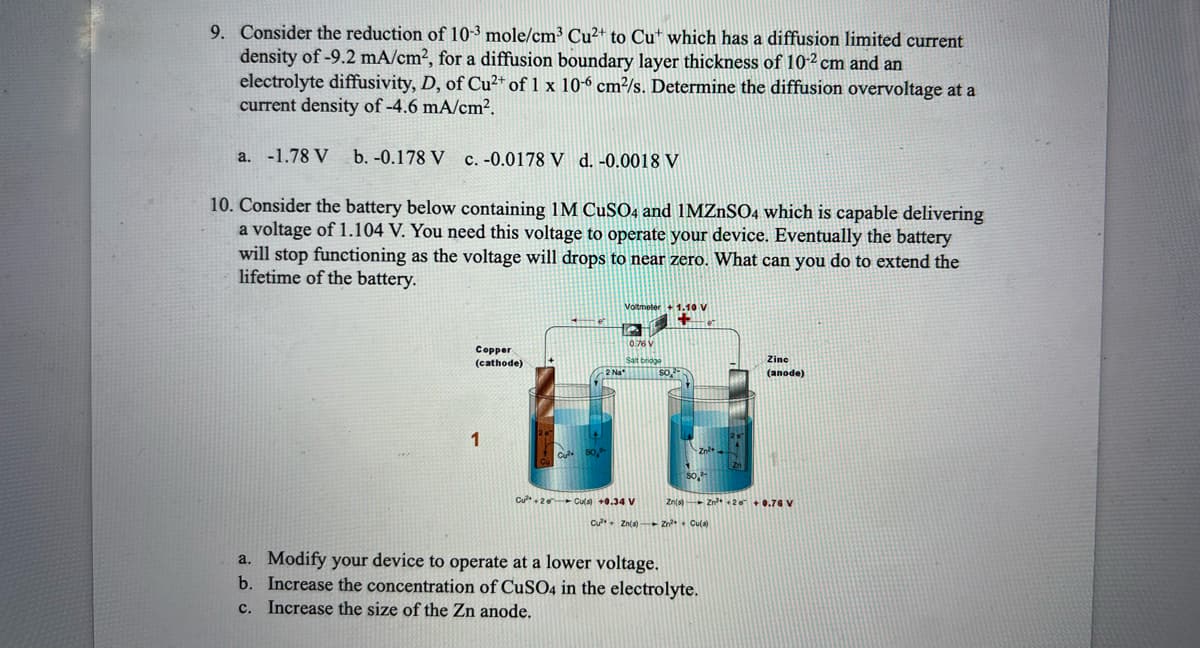
10. Consider the battery below containing 1M CuSO4 and 1MZnSO4 which is capable delivering a voltage of 1.104 V. You need this voltage to operate your device. Eventually the battery will stop functioning as the voltage will drops to near zero. What can you do to extend the lifetime of the battery. Copper (cathode) 1 Cule 2 Na Voltmeter 0.76 V Sat bodge Cu²+2 Cu(s) +0.34 V Cu Zn(a) $0,2 Zn²+ 2n a. Modify your device to operate at a lower voltage. b. Increase the concentration of CuSO4 in the electrolyte. c. Increase the size of the Zn anode. Zine (anode) Zn(s) 2²2 +0.76 V Zn²+ Cu(a)
10. Consider the battery below containing 1M CuSO4 and 1MZnSO4 which is capable delivering a voltage of 1.104 V. You need this voltage to operate your device. Eventually the battery will stop functioning as the voltage will drops to near zero. What can you do to extend the lifetime of the battery. Copper (cathode) 1 Cule 2 Na Voltmeter 0.76 V Sat bodge Cu²+2 Cu(s) +0.34 V Cu Zn(a) $0,2 Zn²+ 2n a. Modify your device to operate at a lower voltage. b. Increase the concentration of CuSO4 in the electrolyte. c. Increase the size of the Zn anode. Zine (anode) Zn(s) 2²2 +0.76 V Zn²+ Cu(a)
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter25: Voltammetry
Section: Chapter Questions
Problem 25.11QAP
Related questions
Question
Solve 10 please
Transcribed Image Text:9. Consider the reduction of 10-3 mole/cm³ Cu²+ to Cut which has a diffusion limited current
density of -9.2 mA/cm², for a diffusion boundary layer thickness of 10-² cm and an
electrolyte diffusivity, D, of Cu²+ of 1 x 10-6 cm²/s. Determine the diffusion overvoltage at a
current density of -4.6 mA/cm².
a. -1.78 V b. -0.178 V c. -0.0178 V d. -0.0018 V
10. Consider the battery below containing 1M CuSO4 and 1MZnSO4 which is capable delivering
a voltage of 1.104 V. You need this voltage to operate your device. Eventually the battery
will stop functioning as the voltage will drops to near zero. What can you do to extend the
lifetime of the battery.
Copper
(cathode)
1
Voltmeter + 1.10 V
Salt bridge
Cu²+2 Cu(s) +0.34 V
Cu²+ Zn(s)
Zine
(anode)
Zn(s) Zn²+20 +0.76 V
Zn²+ + Cu(s)
a. Modify your device to operate at a lower voltage.
b. Increase the concentration of CuSO4 in the electrolyte.
c. Increase the size of the Zn anode.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT