10. The following spectra were acquired from a compound made by dehydration of a secondary alcohol and sulfuric acid, with heat. In the 'H-NMR, the signals at 5.4, 2.0, 1.6 and 1.4 are multiplets, the signal at 0.9 is a triplet. Propose a structure which is consistent with the spectra, and using that structure assign the 'H-nmr and the infrared spectra. L00 assign the characteristic vibrations. 1673 1378 3025 2863 1456 965 2931 4000 3000 2000 1000 KAVENUMBERIl 2H 2H 3H Assign the proton NMR proton nmr (left) integrations: 3H 2H carbon – nmr(below) 132 125 22 14 18 10 8 6 5 3. 2 1ppm o 11 4 200 180 160 140 120 100 80 60 40 20 ppm o 100 - 55 Likely parent ion? Base peak? 80 - Your answer: 40 84 69 20 - 10 20 30 40 50 60 70 80 90 100 m/z Relative Intensity
10. The following spectra were acquired from a compound made by dehydration of a secondary alcohol and sulfuric acid, with heat. In the 'H-NMR, the signals at 5.4, 2.0, 1.6 and 1.4 are multiplets, the signal at 0.9 is a triplet. Propose a structure which is consistent with the spectra, and using that structure assign the 'H-nmr and the infrared spectra. L00 assign the characteristic vibrations. 1673 1378 3025 2863 1456 965 2931 4000 3000 2000 1000 KAVENUMBERIl 2H 2H 3H Assign the proton NMR proton nmr (left) integrations: 3H 2H carbon – nmr(below) 132 125 22 14 18 10 8 6 5 3. 2 1ppm o 11 4 200 180 160 140 120 100 80 60 40 20 ppm o 100 - 55 Likely parent ion? Base peak? 80 - Your answer: 40 84 69 20 - 10 20 30 40 50 60 70 80 90 100 m/z Relative Intensity
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 48AP: The infrared spectrum of the compound with the mass spectrum shown below lacks any significant...
Related questions
Question
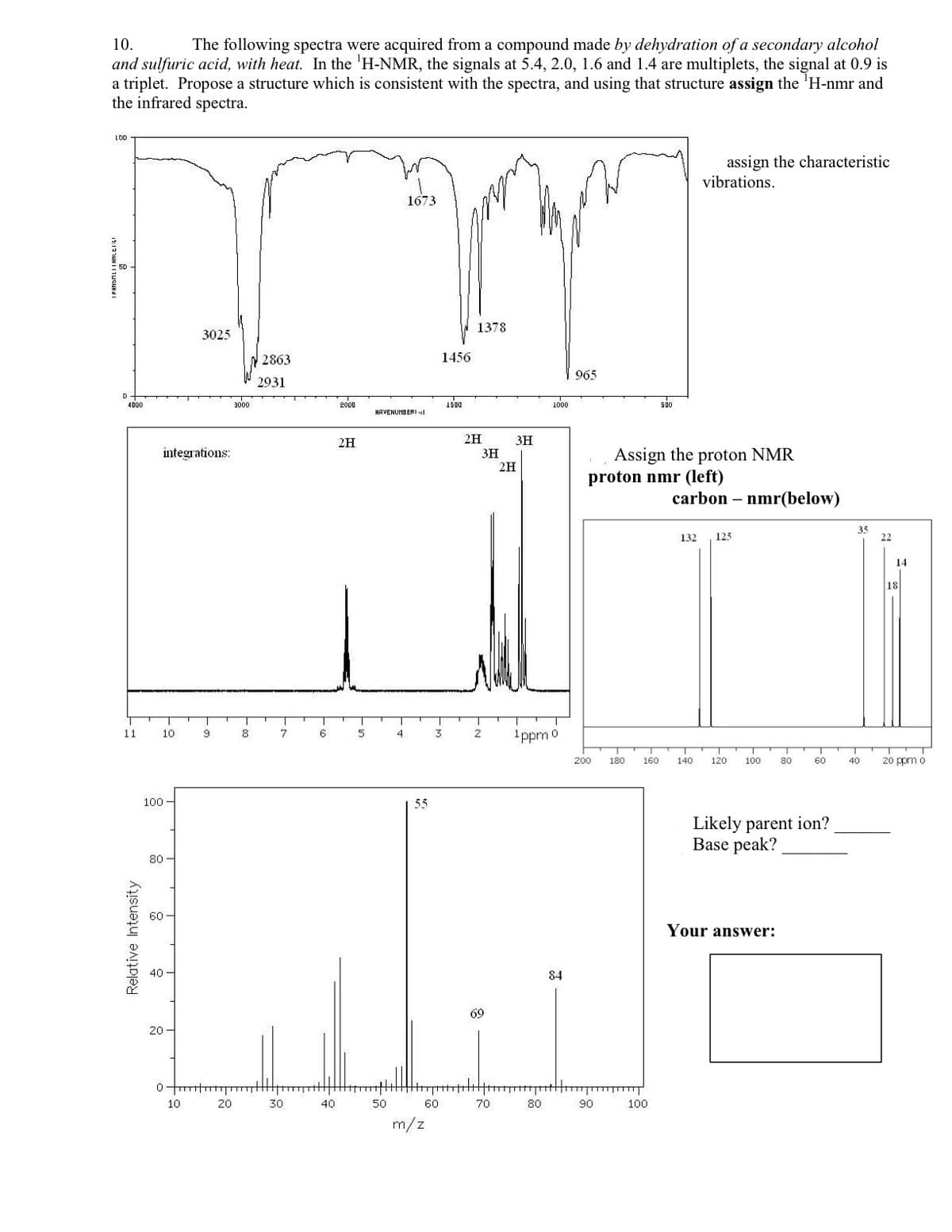
Transcribed Image Text:10.
The following spectra were acquired from a compound made by dehydration of a secondary alcohol
and sulfuric acid, with heat. In the 'H-NMR, the signals at 5.4, 2.0, 1.6 and 1.4 are multiplets, the signal at 0.9 is
a triplet. Propose a structure which is consistent with the spectra, and using that structure assign the 'H-nmr and
the infrared spectra.
LOD
assign the characteristic
vibrations.
1673
1378
3025
2863
1456
965
2931
4000
3000
2000
1500
1000
50
HAVENUMBERI -l
2H
2H
3H
integrations:
3H
2H
Assign the proton NMR
proton nmr (left)
carbon – nmr(below)
35
132
125
22
14
18
11
1ppm 0
10
8
6
4
2
200
180
160
140
120
100
80
60
40
20 ppm o
100
55
Likely parent ion?
Base peak?
80
60
Your answer:
40
84
69
20-
10
20
30
40
50
60
70
80
90
100
m/z
Relative Intensity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole