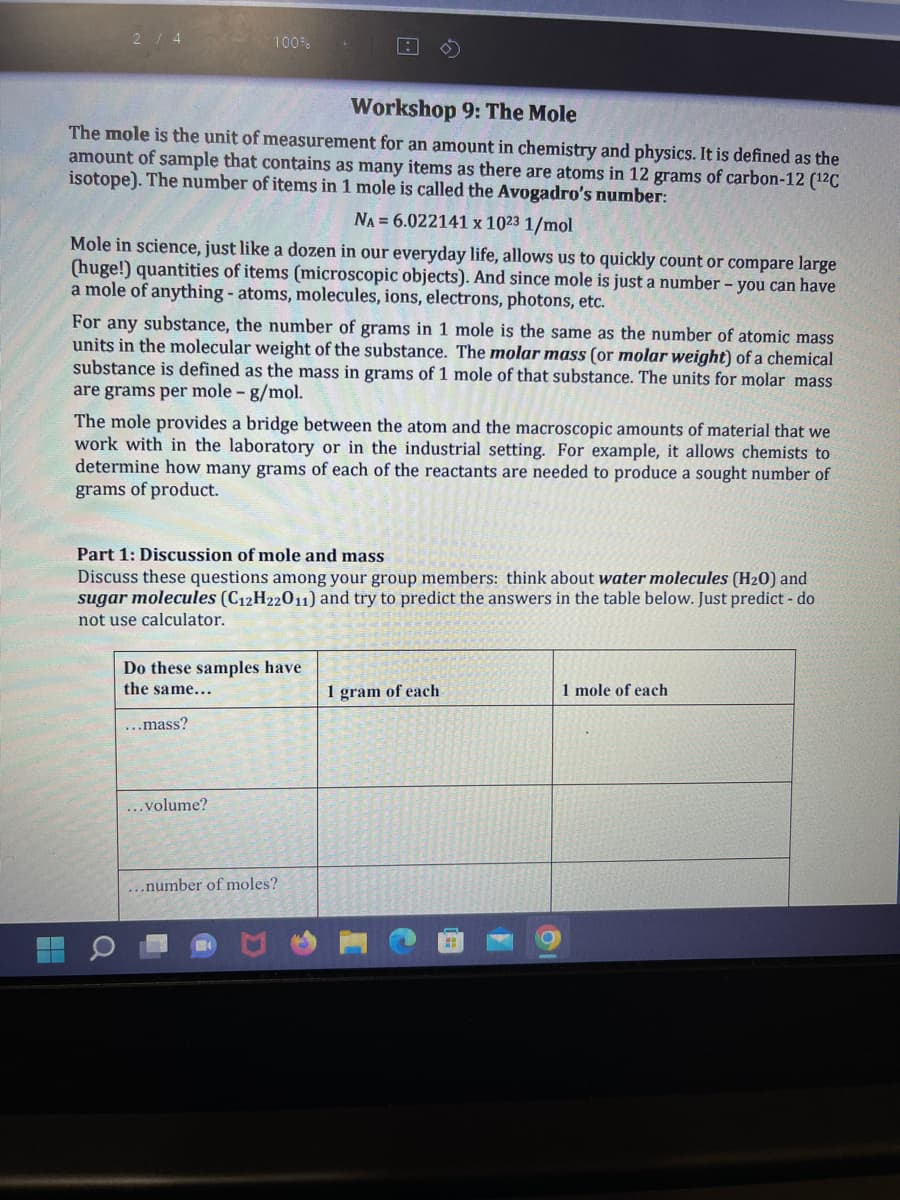
100% Workshop 9: The Mole The mole is the unit of measurement for an amount in chemistry and physics. It is defined as the amount of sample that contains as many items as there are atoms in 12 grams of carbon-12 (¹²C isotope). The number of items in 1 mole is called the Avogadro's number: NA = 6.022141 x 1023 1/mol Mole in science, just like a dozen in our everyday life, allows us to quickly count or compare large (huge!) quantities of items (microscopic objects). And since mole is just a number - you can have a mole of anything - atoms, molecules, ions, electrons, photons, etc. For any substance, the number of grams in 1 mole is the same as the number of atomic mass units in the molecular weight of the substance. The molar mass (or molar weight) of a chemical substance is defined as the mass in grams of 1 mole of that substance. The units for molar mass are grams per mole - g/mol. 1:1 The mole provides a bridge between the atom and the macroscopic amounts of material that we work with in the laboratory or in the industrial setting. For example, it allows chemists to determine how many grams of each of the reactants are needed to produce a sought number of grams of product. Part 1: Discussion of mole and mass Discuss these questions among your group members: think about water molecules (H₂0) and sugar molecules (C12H22011) and try to predict the answers in the table below. Just predict - do not use calculator. Do these samples have the same... ...mass? ...volume? ...number of moles? 1 gram of each HI 1 mole of each
States of Matter
The substance that constitutes everything in the universe is known as matter. Matter comprises atoms which in turn are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction, namely solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Chemical Reactions and Equations
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images









