105. The hydrogen atom contains 1 proton and 1 electron. The radius of the proton is approximately 1.0 fm (femtometer), and the radius of the hydrogen atom is approximately 53 pm (picometers). Calculate the volume of the nucleus and the volume of the atom for hydrogen. What percentage of the hydrogen atom's volume does the nucleus occupy? (Hint: Convert both given radii to m, and then calculate their volumes using the formula for the volume of a sphere, which is V = {ar?.)
105. The hydrogen atom contains 1 proton and 1 electron. The radius of the proton is approximately 1.0 fm (femtometer), and the radius of the hydrogen atom is approximately 53 pm (picometers). Calculate the volume of the nucleus and the volume of the atom for hydrogen. What percentage of the hydrogen atom's volume does the nucleus occupy? (Hint: Convert both given radii to m, and then calculate their volumes using the formula for the volume of a sphere, which is V = {ar?.)
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 34E: Observations of the reaction between nitrogen gas and hydrogen gas show us that 1 volume of nitrogen...
Related questions
Question
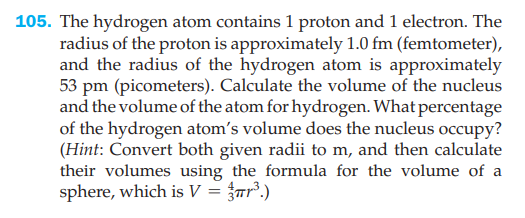
Transcribed Image Text:105. The hydrogen atom contains 1 proton and 1 electron. The
radius of the proton is approximately 1.0 fm (femtometer),
and the radius of the hydrogen atom is approximately
53 pm (picometers). Calculate the volume of the nucleus
and the volume of the atom for hydrogen. What percentage
of the hydrogen atom's volume does the nucleus occupy?
(Hint: Convert both given radii to m, and then calculate
their volumes using the formula for the volume of a
sphere, which is V = {ar?.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning