11 How can society reduce the impact of plastics? use less plastic recycle plastic more often reuse plastic bottles all of the above 0000 For the unbalanced reaction FeO + CO-> CO₂+ Fe, the oxidizing age CO₂ Fe FeO CO th of the following molecules has H-bonding as it strongest intermolecular fo HCI H₂ HBr H₂O
11 How can society reduce the impact of plastics? use less plastic recycle plastic more often reuse plastic bottles all of the above 0000 For the unbalanced reaction FeO + CO-> CO₂+ Fe, the oxidizing age CO₂ Fe FeO CO th of the following molecules has H-bonding as it strongest intermolecular fo HCI H₂ HBr H₂O
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter5: Distillation
Section: Chapter Questions
Problem 5Q
Related questions
Question
100%
Answer all questions in the two pictures please.
Transcribed Image Text:16
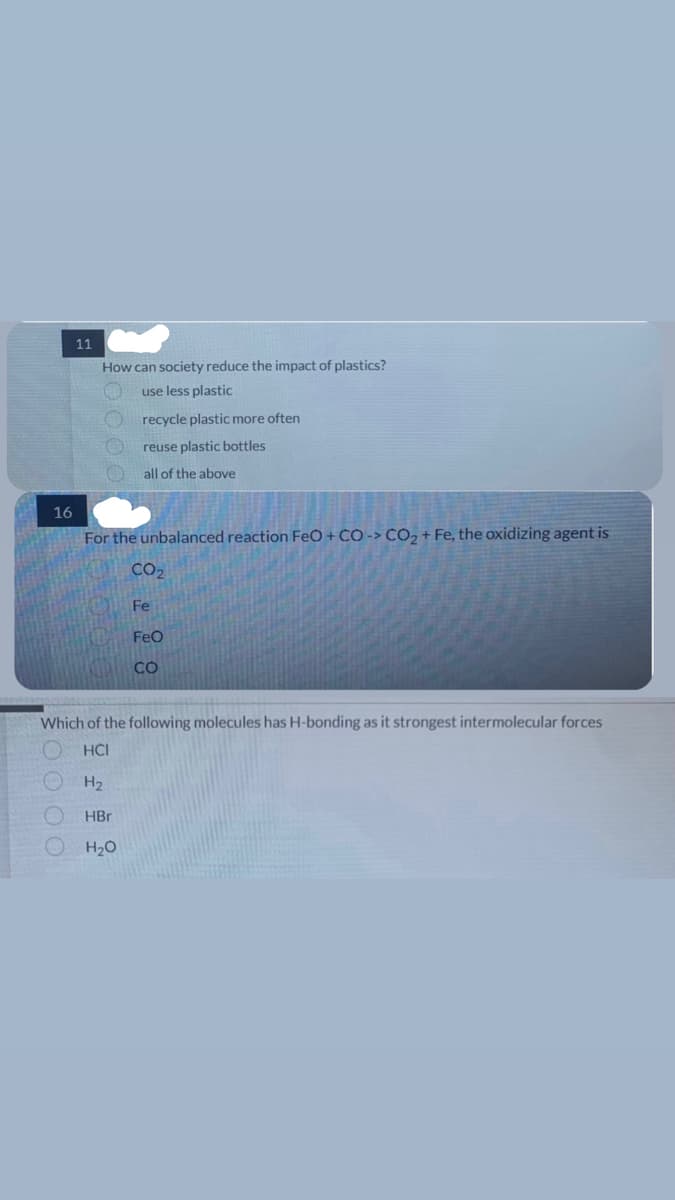
11
00003
How can society reduce the impact of plastics?
use less plastic
recycle plastic more often
reuse plastic bottles
all of the above
For the unbalanced reaction FeO + CO-> CO₂+ Fe, the oxidizing agent is
CO₂
Fe
FeO
CO
Which of the following molecules has H-bonding as it strongest intermolecular forces
HCI
H₂
HBr
H₂O
Transcribed Image Text:00
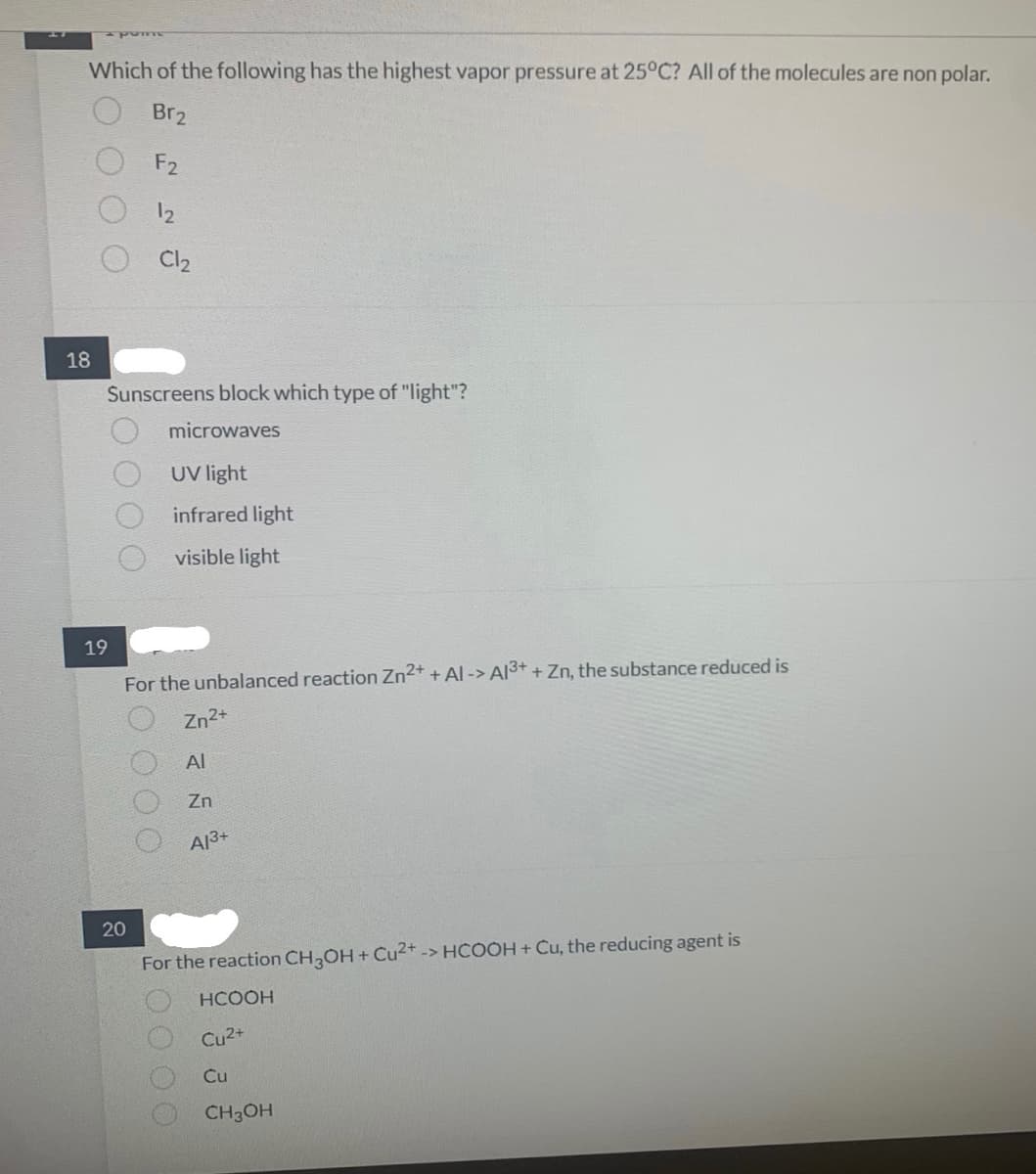
Which of the following has the highest vapor pressure at 25°C? All of the molecules are non polar.
Br₂
F2
18
pome
19
Sunscreens block which type of "light"?
microwaves
UV light
infrared light
visible light
12
20
Cl₂
For the unbalanced reaction Zn²+ + Al-> Al3+ + Zn, the substance reduced is
Zn²+
Al
Zn
A13+
O O O
For the reaction CH3OH + Cu²+ -> HCOOH + Cu, the reducing agent is
O O O O
HCOOH
Cu²+
Cu
CH3OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 8 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
