11. A 101.3-mg sample of an organic compound known to contain Cl is burned in pure O2 and the combustion gases collected in absorbent tubes. The tube used to trap CO2 increases in mass by 167.6 mg, and the tube for trapping H2O shows a 13.7-mg increase. A second sample of 121.8 mg is treated with concentrated HNO3 producing Cl2, which subsequently reacts with Ag*, forming 262.7 mg of AgCl. (MM of C = 12.01 g/mol , O = 16.00 g/mol, CI = 35.45 g/mol, H = 1.008 g/mol, AgCl = 143.32 g/mol). Determine the: %3D
11. A 101.3-mg sample of an organic compound known to contain Cl is burned in pure O2 and the combustion gases collected in absorbent tubes. The tube used to trap CO2 increases in mass by 167.6 mg, and the tube for trapping H2O shows a 13.7-mg increase. A second sample of 121.8 mg is treated with concentrated HNO3 producing Cl2, which subsequently reacts with Ag*, forming 262.7 mg of AgCl. (MM of C = 12.01 g/mol , O = 16.00 g/mol, CI = 35.45 g/mol, H = 1.008 g/mol, AgCl = 143.32 g/mol). Determine the: %3D
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter7: Extraction
Section: Chapter Questions
Problem 1Q
Related questions
Question
11b

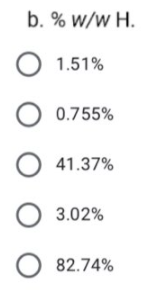
Transcribed Image Text:11. A 101.3-mg sample of an organic compound known to contain Cl is
burned in pure O2 and the combustion gases collected in absorbent
tubes. The tube used to trap CO2 increases in mass by 167.6 mg, and
the tube for trapping H2O shows a 13.7-mg increase. A second sample
of 121.8 mg is treated with concentrated HNO3 producing Cl2, which
subsequently reacts with Ag*, forming 262.7 mg of AgCl. (MM of C =
12.01 g/mol , O = 16.00 g/mol, CI = 35.45 g/mol, H = 1.008 g/mol, AgCl =
143.32 g/mol). Determine the:
%3D
Transcribed Image Text:b. % w/w H.
1.51%
O 0.755%
O 41.37%
O 3.02%
O 82.74%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT