The frequency factor in the Arrhenius equation can best be described as O a. a factor which corrects for the conversion between J and kJ. O b.a numerical description of the amount of energy needed by colliding reactant molecules in order to form products. Oc the probability that a collision between molecules will have the corect relative orientation for reaction to occur. d. a numerical description of the amount of energy released by colliding reactant molecules when they form products. e. a numerical description of how often molecules collide with the proper orientation to react ar a specific concentration.
The frequency factor in the Arrhenius equation can best be described as O a. a factor which corrects for the conversion between J and kJ. O b.a numerical description of the amount of energy needed by colliding reactant molecules in order to form products. Oc the probability that a collision between molecules will have the corect relative orientation for reaction to occur. d. a numerical description of the amount of energy released by colliding reactant molecules when they form products. e. a numerical description of how often molecules collide with the proper orientation to react ar a specific concentration.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.66PAE: The recycling of polymers represents only one industrial process that allows creating order in one...
Related questions
Question
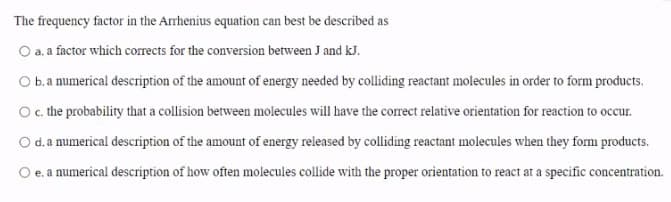
Transcribed Image Text:The frequency factor in the Arrhenius equation can best be described as
O a. a factor which corrects for the conversion between J and kJ.
O b.a numerical description of the amount of energy needed by colliding reactant molecules in order to form products.
Oc the probability that a collision between molecules will have the corect relative orientation for reaction to occur.
d.a numerical description of the amount of energy released by colliding reactant molecules when they form products.
e. a numerical description of how often molecules collide with the proper orientation to react ar a specific concentration.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning