11. For each of the following questions, refer to the phase diagram for a mysterlous compound X Phase diagram for mysterious compound X 100 90 80 liquid 70 solid 60 50 40 gas 30 20 10 -100 0 100 200 300 400 500 600 700 800 Temperature (degrees Celsius) (a) What Is the critical temperature of compound X? (b) If you were to have a bottle containing compound X In your closet, what phase would It most likely be in? (c) At what temperature and pressure will all the three phases coexist (d) If i have a bottle of compound X at a pressure of 45 atm and temperature at 100 °C, what will happen if I ralse the temperature to 400 °C? (e) Why can't compound X be botled at a temperature of 200 °C? (f) If I wanted to, could I drink compound X Pressure (atm)
11. For each of the following questions, refer to the phase diagram for a mysterlous compound X Phase diagram for mysterious compound X 100 90 80 liquid 70 solid 60 50 40 gas 30 20 10 -100 0 100 200 300 400 500 600 700 800 Temperature (degrees Celsius) (a) What Is the critical temperature of compound X? (b) If you were to have a bottle containing compound X In your closet, what phase would It most likely be in? (c) At what temperature and pressure will all the three phases coexist (d) If i have a bottle of compound X at a pressure of 45 atm and temperature at 100 °C, what will happen if I ralse the temperature to 400 °C? (e) Why can't compound X be botled at a temperature of 200 °C? (f) If I wanted to, could I drink compound X Pressure (atm)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 19QAP
Related questions
Question
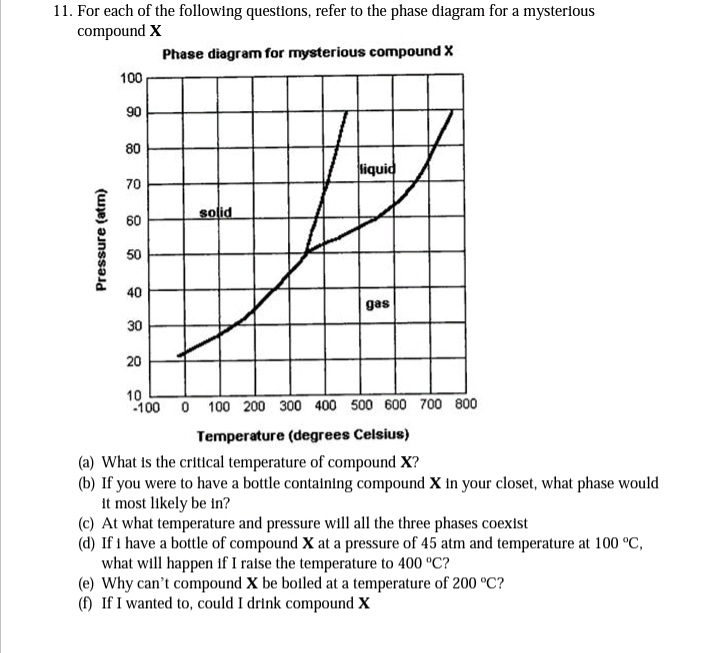
Transcribed Image Text:11. For each of the following questions, refer to the phase diagram for a mysterious
compound X
Phase diagram for mysterious compound X
100
90
80
liquid
70
solid
60
50
40
gas
30
20
100 0 100 200 300 400 500 600 700 800
Temperature (degrees Celsius)
(a) What is the critical temperature of compound X?
(b) If you were to have a bottle contatning compound X in your closet, what phase would
it most likely be in?
(c) At what temperature and pressure will all the three phases coexist
(d) If 1 have a bottle of compound X at a pressure of 45 atm and temperature at 100 °C,
what will happen if I raise the temperature to 400 °C?
(e) Why can't compound X be boiled at a temperature of 200 °C?
(f) If I wanted to, could I drink compound X
Pressure (atm)
8 8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
