10000 1000 CO, sod 100 co, que Somaon pont 10 -70.SCati co, gas 0001 140 -120 -10O 00 60 40 -20 0 20 40 60 80 100 Temperature eC) If I had a quantity of this substance at a pressure of 100 amt and a temperature of -80 degree Celsius and raised the Temperature to -40 Degrees Celsius, what phase change(s) would occur ? Solidification O Fusion Condensation O Vaporization Pressure (atm)
10000 1000 CO, sod 100 co, que Somaon pont 10 -70.SCati co, gas 0001 140 -120 -10O 00 60 40 -20 0 20 40 60 80 100 Temperature eC) If I had a quantity of this substance at a pressure of 100 amt and a temperature of -80 degree Celsius and raised the Temperature to -40 Degrees Celsius, what phase change(s) would occur ? Solidification O Fusion Condensation O Vaporization Pressure (atm)
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 38GQ: The following data are the equilibrium vapor pressure of limonene, C10H16, at various temperatures....
Related questions
Question
100%
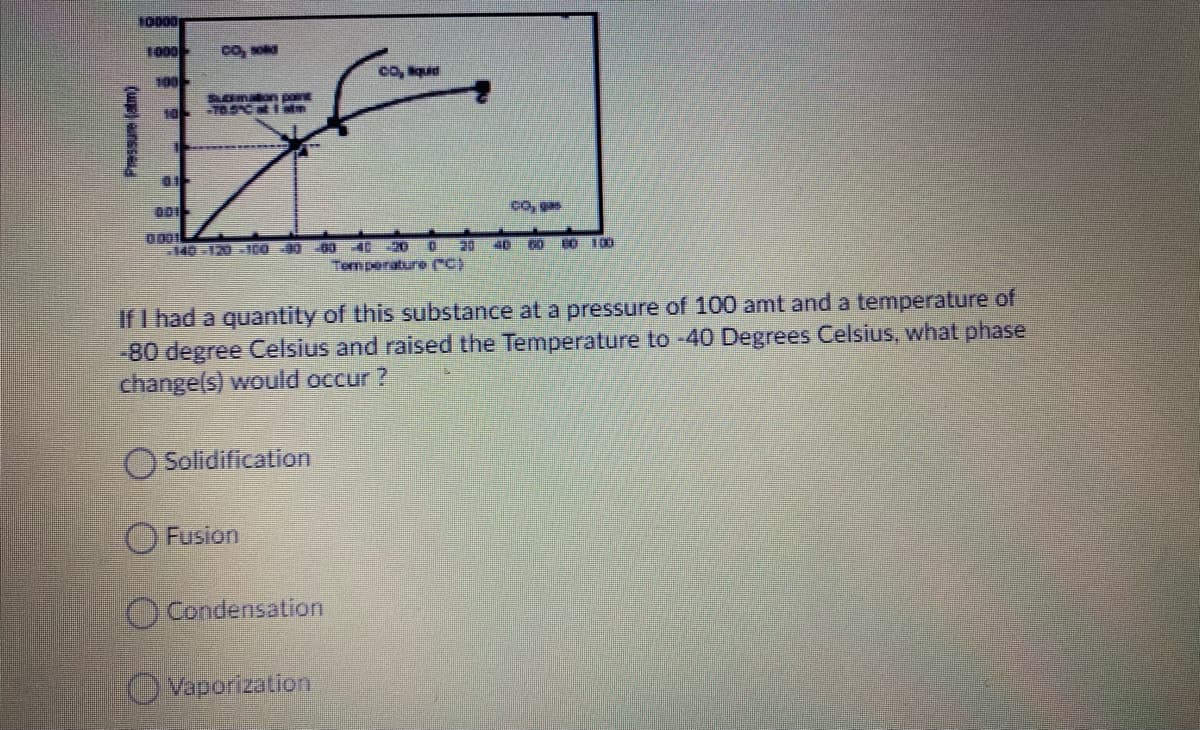
Transcribed Image Text:10000
1000
co, soa
100
co, qud
Somaon pon
10
000
140-120100 0 60 402D O
CO 100
Tem perature C)
If I had a quantity of this substance at a pressure of 100 amt and a temperature of
-80 degree Celsius and raised the Temperature to -40 Degrees Celsius, what phase
change(s) would occur ?
Solidification
O Fusion
Condensation
Vaporization
(up) aunsse
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning