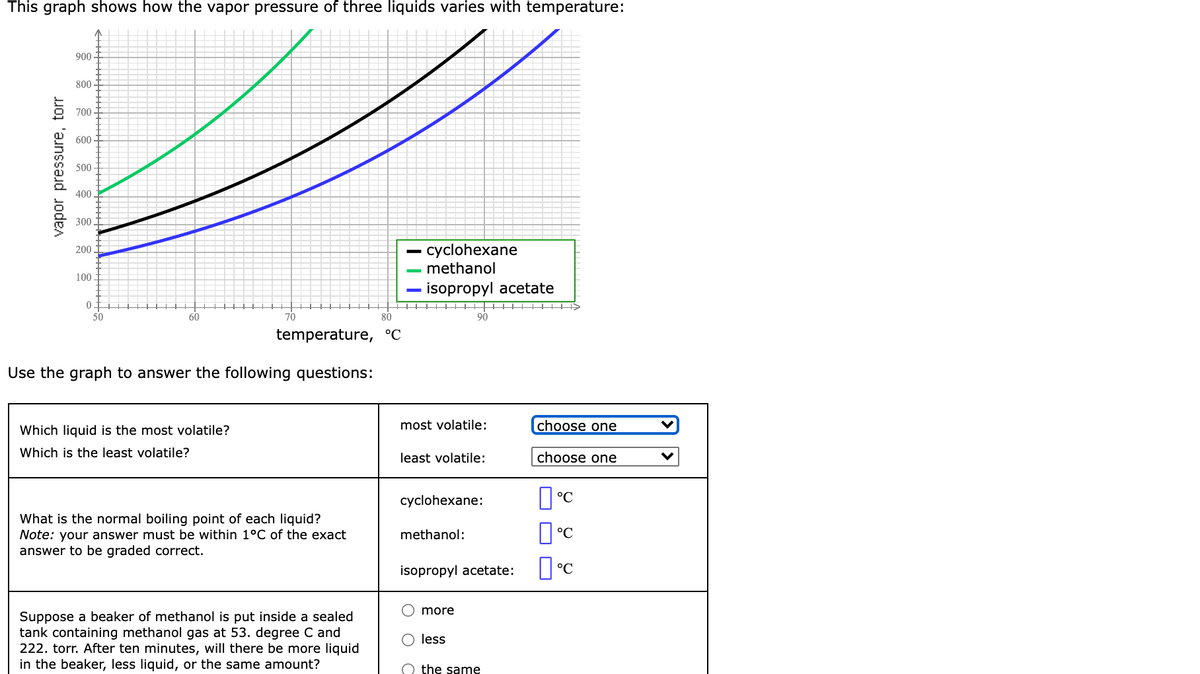
This graph shows how the vapor pressure of three liquids varies with temperature: 900 800 700 600 500 400 300t - cyclohexane - methanol - isopropyl acetate 200 100 0 50 tII tt+ 60 90 temperature, °C Use the graph to answer the following questions: Which liquid is the most volatile? most volatile: choose one Which is the least volatile? least volatile: choose one cyclohexane: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. methanol: °C isopropyl acetate: °C O more Suppose a beaker of methanol is put inside a sealed tank containing methanol gas at 53. degree C and 222. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? O less O the same vapor pressure, torr
States of Matter
The substance that constitutes everything in the universe is known as matter. Matter comprises atoms which in turn are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction, namely solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Chemical Reactions and Equations
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









