1012 VII VI 10° A Liquid 10 Vapour 10 1 200 400 600 800 Temperature, T/K Figure 4A.9 The phase diagram for water showing the different solid phases, which are indicated with Roman numerals I, ,..; solid phase I (ice I) is ordinary ice. The path ABCD is discussed in Brief illustration 4A.7. Pressure, p/Pa
1012 VII VI 10° A Liquid 10 Vapour 10 1 200 400 600 800 Temperature, T/K Figure 4A.9 The phase diagram for water showing the different solid phases, which are indicated with Roman numerals I, ,..; solid phase I (ice I) is ordinary ice. The path ABCD is discussed in Brief illustration 4A.7. Pressure, p/Pa
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.50E
Related questions
Question
Refer to Fig. 4A.9. Which phase or phases would you expect to be present for a sample of H2O at: (i) 100 K and 1 atm; (ii) 300 K and 10 atm; (iii) 273.16 K and 611 Pa?
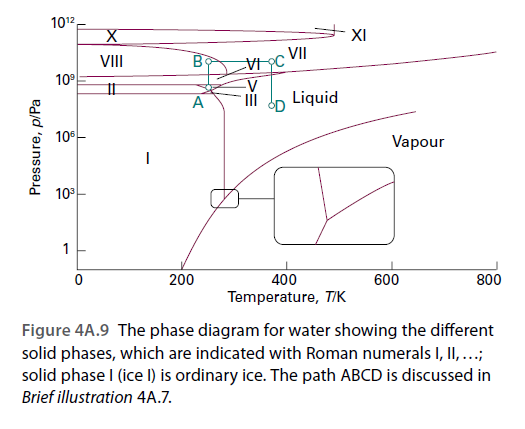
Transcribed Image Text:1012
VII
VI
10°
A
Liquid
10
Vapour
10
1
200
400
600
800
Temperature, T/K
Figure 4A.9 The phase diagram for water showing the different
solid phases, which are indicated with Roman numerals I, ,..;
solid phase I (ice I) is ordinary ice. The path ABCD is discussed in
Brief illustration 4A.7.
Pressure, p/Pa
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT