11. Refer to the solubility curve SOLUBILITY (g/100 g water) 160 140 120 100 80 0 20 40 80 80 Temp (°C) a) What is the solubility of b) At what temperature will saturated solution? c) What is the maximum n water at 30°C? d) Specifically describe wh solution containing 150
11. Refer to the solubility curve SOLUBILITY (g/100 g water) 160 140 120 100 80 0 20 40 80 80 Temp (°C) a) What is the solubility of b) At what temperature will saturated solution? c) What is the maximum n water at 30°C? d) Specifically describe wh solution containing 150
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 99E: The equation for a reaction by which a solution of sodium carbonate may be standardized is...
Related questions
Question
Please send me the question in 30 minutes it's very urgent plz
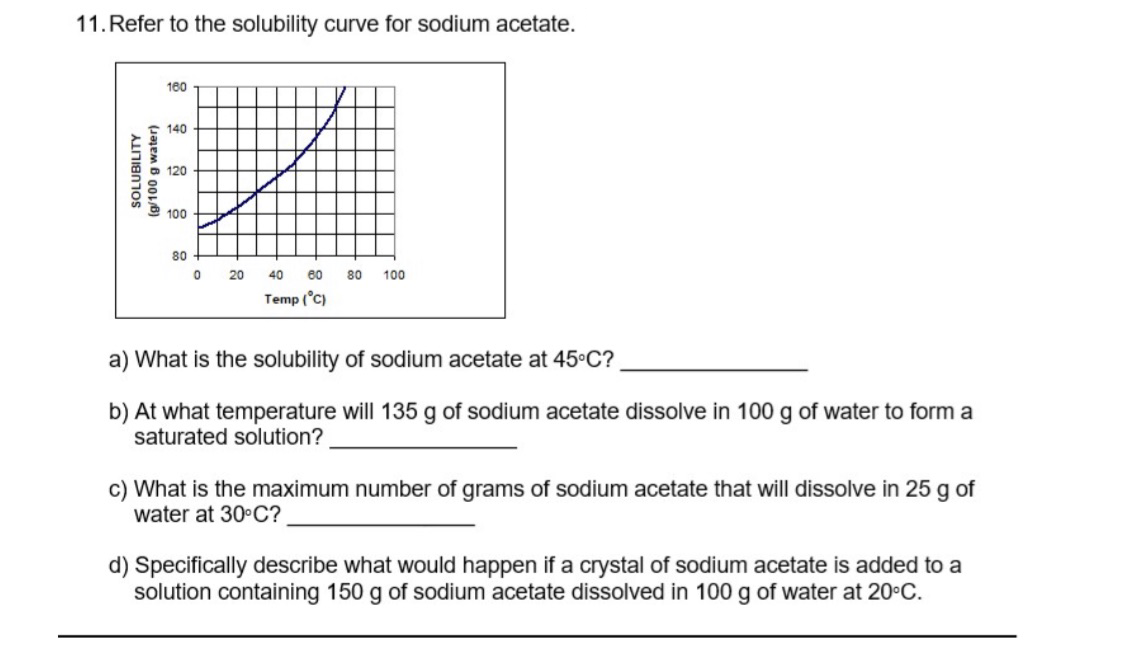
Transcribed Image Text:11. Refer to the solubility curve for sodium acetate.
SOLUBILITY
(g/100 g water)
160
140
120
100
80
0 20
40 60
Temp (°C)
80 100
a) What is the solubility of sodium acetate at 45°C?
b) At what temperature will 135 g of sodium acetate dissolve in 100 g of water to form a
saturated solution?
c) What is the maximum number of grams of sodium acetate that will dissolve in 25 g of
water at 30°C?
d) Specifically describe what would happen if a crystal of sodium acetate is added to a
solution containing 150 g of sodium acetate dissolved in 100 g of water at 20°C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning