12. Below are the processes that describe the first, second, and third ionization energies of an aluminium atom Which process would require the least amount of energy and wh y? 1st Al (g)-Art(g) +e 2nd Art(g) - AR+ (g) + e 3rd ARtlg) -- A (g) + e OA The 1st jonization energy because it's more attracted to the nucleus DEThe 1st jonization energy because it's less attracted to the nucleus CThe 2nd ionization energy because it's more attracted to the nucleus The 3rd jonization energy because it's least attracted to the nucleus
12. Below are the processes that describe the first, second, and third ionization energies of an aluminium atom Which process would require the least amount of energy and wh y? 1st Al (g)-Art(g) +e 2nd Art(g) - AR+ (g) + e 3rd ARtlg) -- A (g) + e OA The 1st jonization energy because it's more attracted to the nucleus DEThe 1st jonization energy because it's less attracted to the nucleus CThe 2nd ionization energy because it's more attracted to the nucleus The 3rd jonization energy because it's least attracted to the nucleus
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter6: The Periodic Table And Atomic Structure
Section: Chapter Questions
Problem 6.67PAE
Related questions
Question
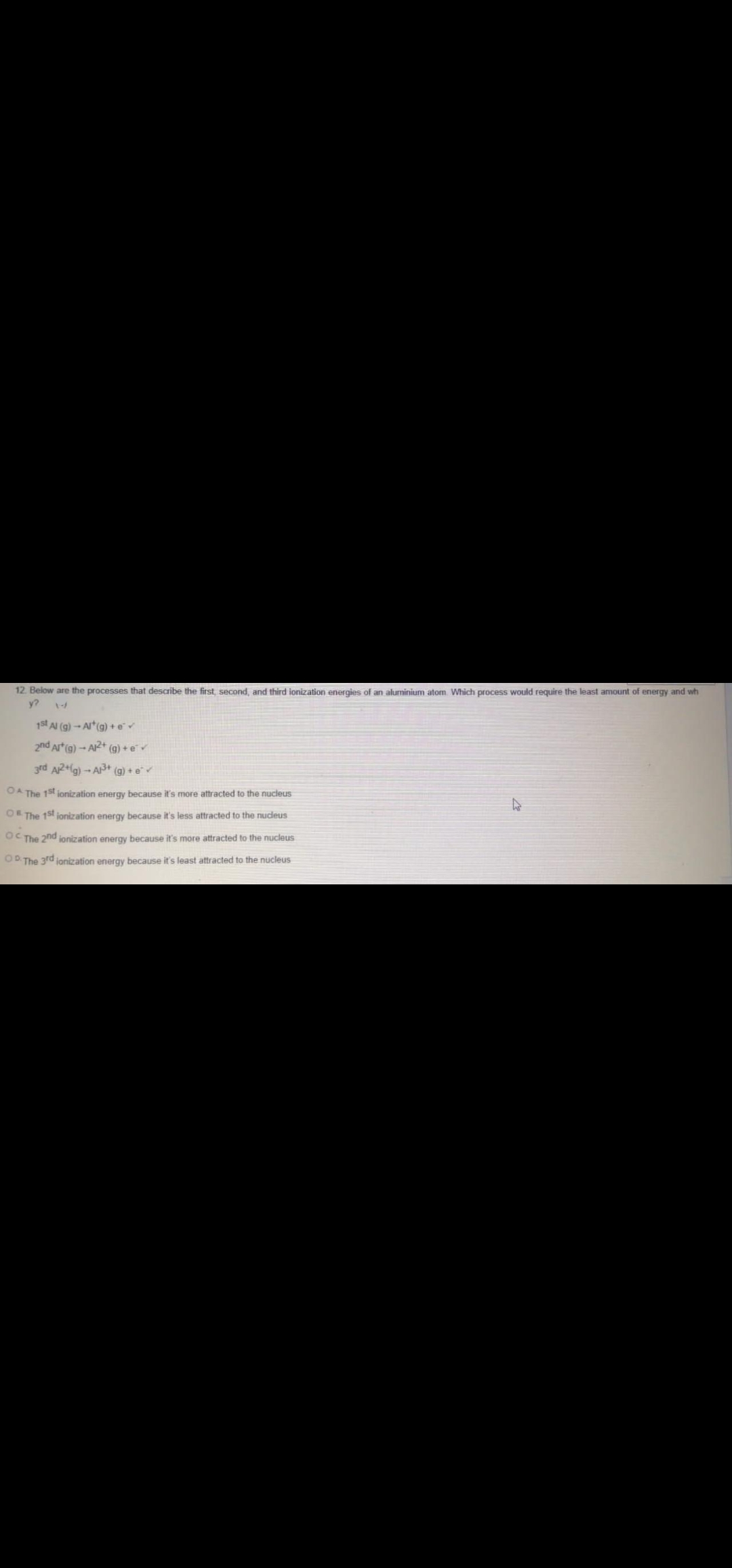
Transcribed Image Text:12. Below are the processes that describe the first, second, and third ionization energies of an aluminium atom Which process would require the least amount of energy and wh
y?
1st Al (g) - Art(g) +e
2nd Art(g) - A2+ (g) + e
3rd A2+la) - A+ (g) + e
OA The 1st jonization energy because it's more attracted to the nucleus
OE The 15t jonization energy because it's less attracted to the nucleus
OC The 2nd ionization energy because it's more attracted to the nucleus
OB The 3rd jonization energy because it's least attracted to the nucleus
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning