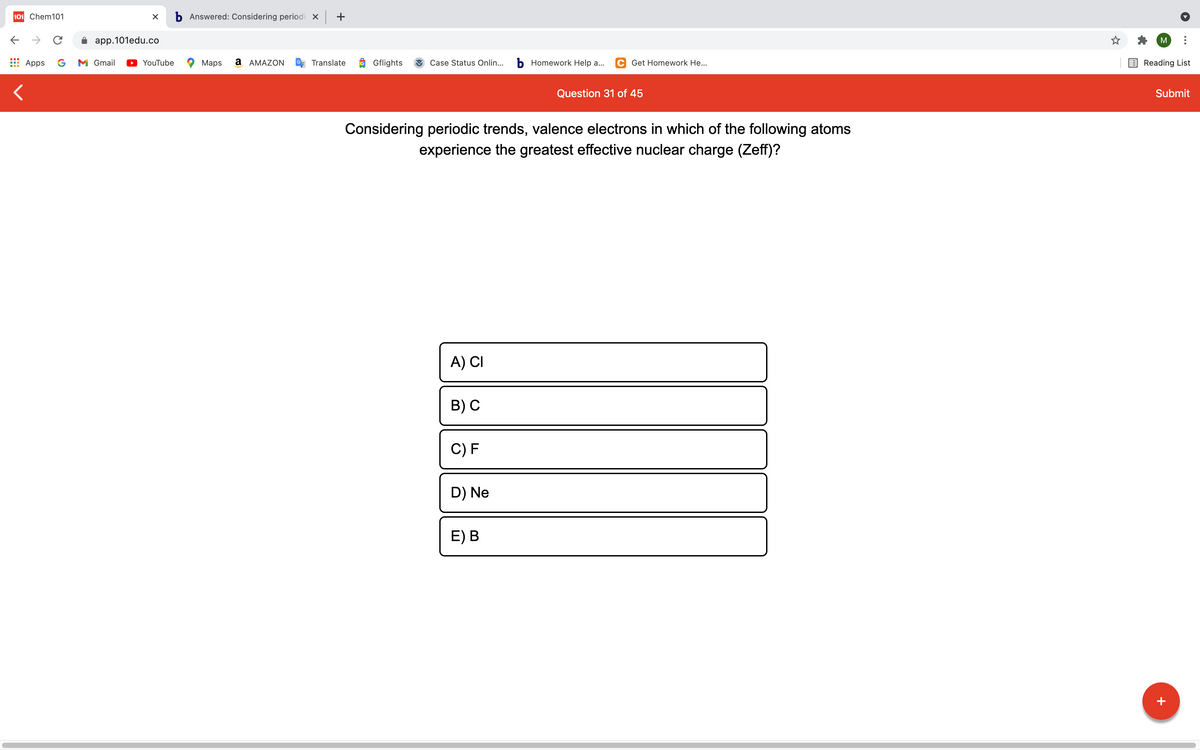
Considering periodic trends, valence electrons in which of the following atoms experience the greatest effective nuclear charge (Zeff)? A) CI B) C C) F D) Ne E) B
Considering periodic trends, valence electrons in which of the following atoms experience the greatest effective nuclear charge (Zeff)? A) CI B) C C) F D) Ne E) B
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 33PS: Identify the element that corresponds to each of the simplified photoelectron spectral data given...
Related questions
Question
Option A is not the right answer. I received option A as the correct choice yesterday but it was wrong. Thanks
Transcribed Image Text:101 Chem101
b Answered: Considering periodi x +
->
app.101edu.co
M
Apps
G
M Gmail
YouTube
Maps
а АМAZON
Translate
O Gflights
Case Status Onlin...
b Homework Help a...
C Get Homework He...
Reading List
Question 31 of 45
Submit
Considering periodic trends, valence electrons in which of the following atoms
experience the greatest effective nuclear charge (Zeff)?
A) CI
B) С
C) F
D) Ne
E) В
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning