12. Using the table below, determine AGrn for a spontaneous redox reaction between lead and cadmium (in units of kJ/mol) rxn Standard reduction potential (V) Half-reaction Cd2+(aq)2e Cd (s) Ph2+ (aq) 2e = Pb (s) 0.40 -0.13 (а) -26 (b) -52 (d) Can't tell /need more info (c) +27
12. Using the table below, determine AGrn for a spontaneous redox reaction between lead and cadmium (in units of kJ/mol) rxn Standard reduction potential (V) Half-reaction Cd2+(aq)2e Cd (s) Ph2+ (aq) 2e = Pb (s) 0.40 -0.13 (а) -26 (b) -52 (d) Can't tell /need more info (c) +27
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 43E: Consider the following metals: Ag, Au, Mg, Ni, and Zn. Which of these metals could be used as a...
Related questions
Question
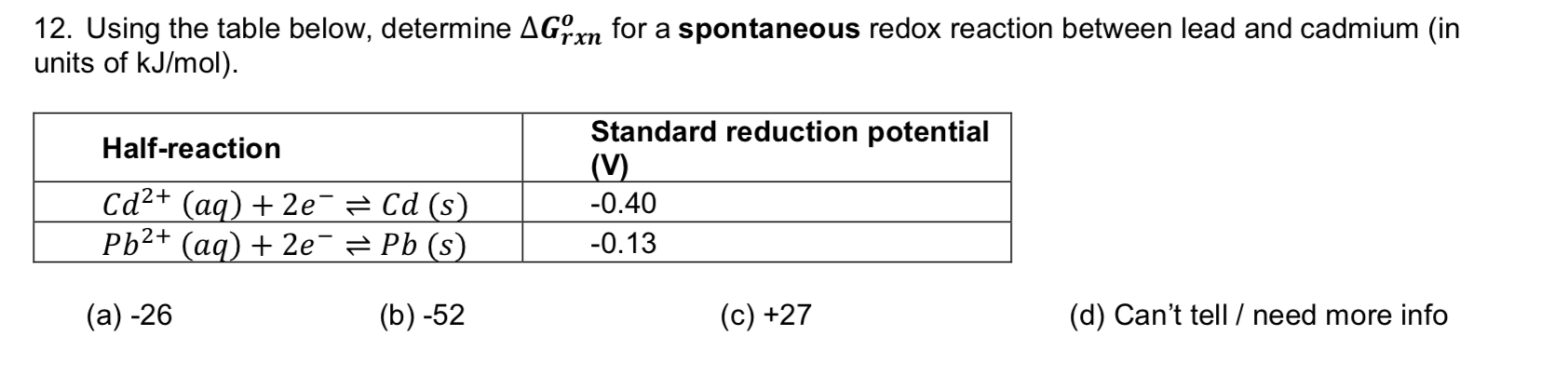
Transcribed Image Text:12. Using the table below, determine AGrn for a spontaneous redox reaction between lead and cadmium (in
units of kJ/mol)
rxn
Standard reduction potential
(V)
Half-reaction
Cd2+(aq)2e Cd (s)
Ph2+ (aq) 2e = Pb (s)
0.40
-0.13
(а) -26
(b) -52
(d) Can't tell /need more info
(c) +27
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning