3. write a report on the use of zinc as a “sacrificial anode” to minimise the corrosion of iron and steel such as on ships. Use the standard electrode potentials given in question 2 above to predict the cell emf and overall equation for the reaction that would occur in standard conditions between zinc and iron. With ocean-going ships, however, what is the solution in which the two metals are immersed? Is this solution always at an exact concentration of 1 mol dm-3? And in what ways does this real-life situation differ from the standard condition of 25oC / 298K Picture of question 2 is also given
3. write a report on the use of zinc as a “sacrificial anode” to minimise the corrosion of iron and steel such as on ships. Use the standard electrode potentials given in question 2 above to predict the cell emf and overall equation for the reaction that would occur in standard conditions between zinc and iron. With ocean-going ships, however, what is the solution in which the two metals are immersed? Is this solution always at an exact concentration of 1 mol dm-3? And in what ways does this real-life situation differ from the standard condition of 25oC / 298K Picture of question 2 is also given
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter23: Potentiometry
Section: Chapter Questions
Problem 23.12QAP: What arc the advantages of microfabricated ISEs? Describe typical applications of this type of...
Related questions
Question
3. write a report on the use of zinc as a “sacrificial anode” to minimise the corrosion of iron and steel such as on ships. Use the standard electrode potentials given in question 2 above to predict the cell emf and overall equation for the reaction that would occur in standard conditions between zinc and iron. With ocean-going ships, however, what is the solution in which the two metals are immersed? Is this solution always at an exact concentration of 1 mol dm-3? And in what ways does this real-life situation differ from the standard condition of 25oC / 298K
Picture of question 2 is also given
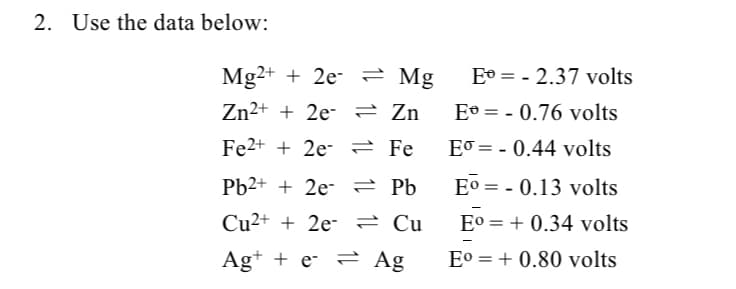
Transcribed Image Text:2. Use the data below:
Mg2+ + 2e- = Mg
E° = - 2.37 volts
Zn²+ + 2e- = Zn
Eº = - 0.76 volts
Fe2+ + 2e- 2 Fe
Eo = - 0.44 volts
Pb2+ + 2e- 2 Pb
E° = - 0.13 volts
Cu2+ + 2e- 2 Cụ
E° = + 0.34 volts
%3D
Ag+ + e- = Ag
E° = + 0.80 volts
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT