Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter18: Aromaticity
Section: Chapter Questions
Problem 11E
Related questions
Question
100%
can you solve 12.66. Devise a synthesis of A from the three strating materials given. You may use any other needed organic or inorganic reagents
Transcribed Image Text:b.
HO.
HO.
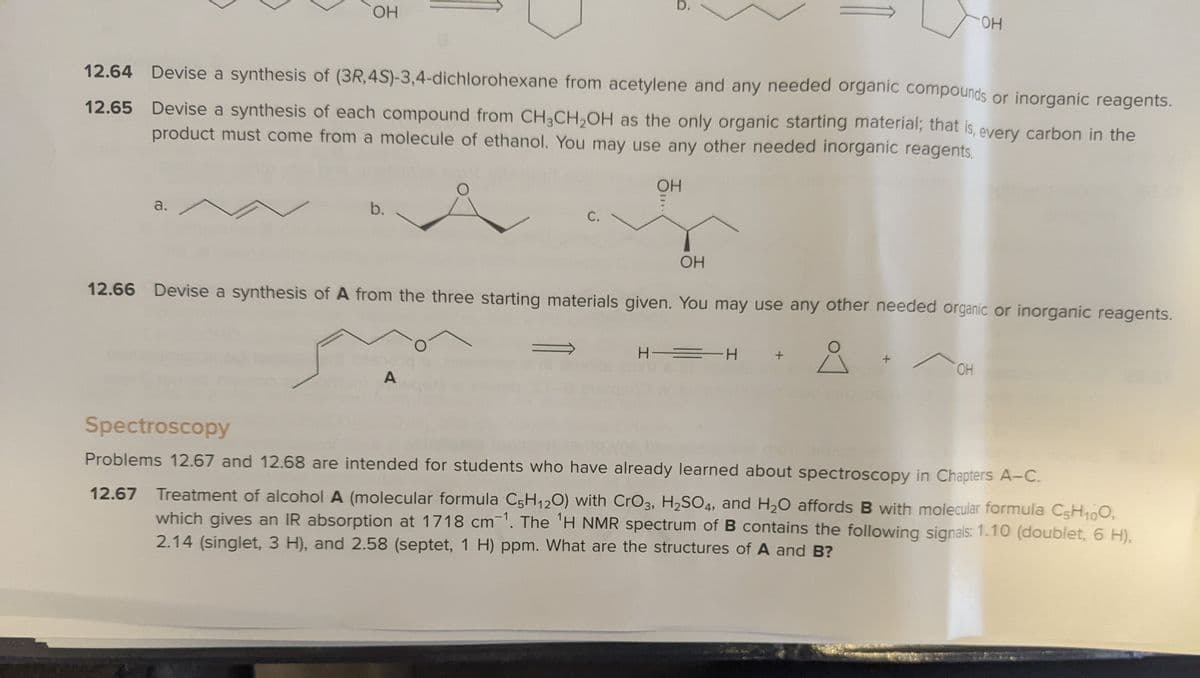
12.64 Devise a synthesis of (3R,4S)-3,4-dichlorohexane from acetylene and any needed organic compounds or inorganic reagents.
12.65 Devise a synthesis of each compound from CH3CH,OH as the only organic starting material; that is, every carbon in the
product must come from a molecule of ethanol. You may use any other needed inorganic reagents.
a.
b.
С.
OH
12.66 Devise a synthesis of A from the three starting materials given. You may use any other needed organic or inorganic reagents.
H = H
HO.
A
Spectroscopy
Problems 12.67 and 12.68 are intended for students who have already learned about spectroscopy in Chapters A-C.
12.67 Treatment of alcohol A (molecular formula C5H120) with CrO3, H2SO4, and H20 affords B with molecular formula C5H100,
which gives an IR absorption at 1718 cm'. The 'H NMR spectrum of B contains the following signals: 1.10 (doublet, 6 H),
2.14 (singlet, 3 H), and 2.58 (septet, 1 H) ppm. What are the structures of A and B?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
