1:26 PM Online teaching and X now.com/ilrn/takeAssignment/takeCovalentActivity.do?locator assignment-take&takeAssignmentSessionLocator ags Review Topics] References Use the References to access important values if needed for this question. The acid ionization constant for Cu(H20)6 (aq) is 1.0x10. Calculate the pH ofa 0.0208 M solution of 2+ Cu(NO3)2 pH = Submit Answer Retry Entire Group 4 more group attempts remaining Visited Next O verizon 1:26 PM aching and X m/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take&takeAssignmentSessionLocator Review Topics References Use the References to access important values if needed for this question. The acid ionization constant for Cu(H0)6 (aq) is 1.0x10. Calculate the pH of a 0.0316 M solution of Cu(NO3)2 pH Retry Entire Group 4 more group attempts remaining Submit Answer Next Previous 4
1:26 PM Online teaching and X now.com/ilrn/takeAssignment/takeCovalentActivity.do?locator assignment-take&takeAssignmentSessionLocator ags Review Topics] References Use the References to access important values if needed for this question. The acid ionization constant for Cu(H20)6 (aq) is 1.0x10. Calculate the pH ofa 0.0208 M solution of 2+ Cu(NO3)2 pH = Submit Answer Retry Entire Group 4 more group attempts remaining Visited Next O verizon 1:26 PM aching and X m/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take&takeAssignmentSessionLocator Review Topics References Use the References to access important values if needed for this question. The acid ionization constant for Cu(H0)6 (aq) is 1.0x10. Calculate the pH of a 0.0316 M solution of Cu(NO3)2 pH Retry Entire Group 4 more group attempts remaining Submit Answer Next Previous 4
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter15: Acids And Bases
Section: Chapter Questions
Problem 15.127QP: A solution contains 4.25g of ammonia per 250.0 mL of solution. Electrical conductivity measurements...
Related questions
Question
![1:26 PM
Online teaching and
X
now.com/ilrn/takeAssignment/takeCovalentActivity.do?locator assignment-take&takeAssignmentSessionLocator
ags
Review Topics]
References
Use the References to access important values if needed for this question.
The acid ionization constant for Cu(H20)6 (aq) is 1.0x10. Calculate the pH ofa 0.0208 M solution of
2+
Cu(NO3)2
pH =
Submit Answer
Retry Entire Group
4 more group attempts remaining
Visited
Next
O
verizon](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F3970f737-304f-4661-a544-bfa2aa74a34f%2Fd20aa7cf-dc6b-4a61-bf70-95b2e4db67ec%2Fdnxcp9.jpeg&w=3840&q=75)
Transcribed Image Text:1:26 PM
Online teaching and
X
now.com/ilrn/takeAssignment/takeCovalentActivity.do?locator assignment-take&takeAssignmentSessionLocator
ags
Review Topics]
References
Use the References to access important values if needed for this question.
The acid ionization constant for Cu(H20)6 (aq) is 1.0x10. Calculate the pH ofa 0.0208 M solution of
2+
Cu(NO3)2
pH =
Submit Answer
Retry Entire Group
4 more group attempts remaining
Visited
Next
O
verizon
Transcribed Image Text:1:26 PM
aching and X
m/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take&takeAssignmentSessionLocator
Review Topics
References
Use the References to access important values if needed for this question.
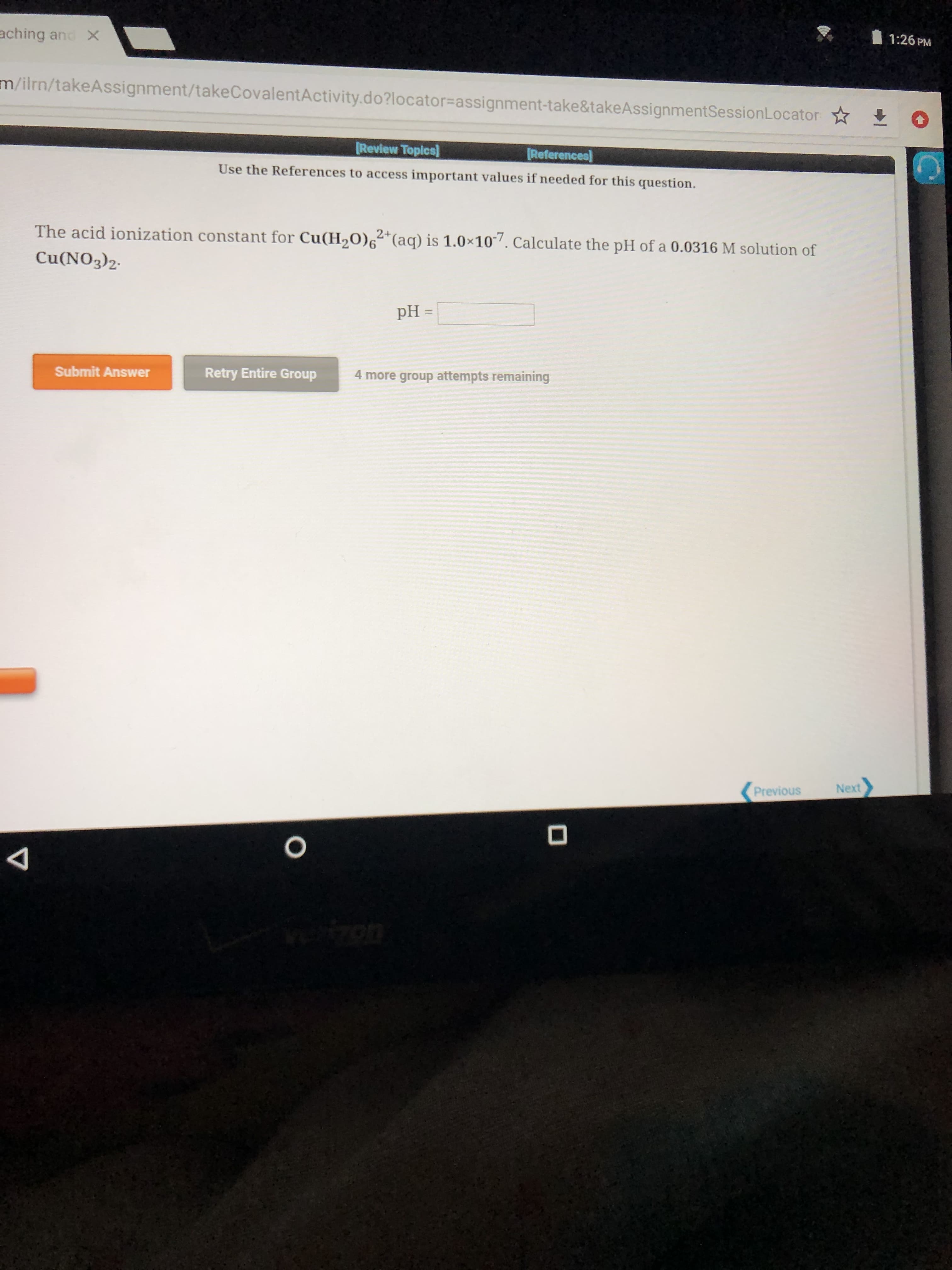
The acid ionization constant for Cu(H0)6 (aq) is 1.0x10. Calculate the pH of a 0.0316 M solution of
Cu(NO3)2
pH
Retry Entire Group
4 more group attempts remaining
Submit Answer
Next
Previous
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning