13. Which acid in each pair below is the stronger acid? Please explain your answer with the structural considerations presented in class - EWG strength, number of EWGS, or proximity of EWGS. Br Br Explanaation: or н,с EWG strength number of EWGs proximity of EWGS Br он он bromoacetie acid 2-dibromoacetic acid Explanation: сн, снс CH2C EWG strength number of EWGS proximity of EWGS or Он Он 3-fluoropropanoic acid 2-fluoropropanoie seid Choose the more acidic proton from each mo lecule, i.e., each molecule has two acidic protons. which is more acidic? which is more acidic? он но но Он Br Br Why? Why?
13. Which acid in each pair below is the stronger acid? Please explain your answer with the structural considerations presented in class - EWG strength, number of EWGS, or proximity of EWGS. Br Br Explanaation: or н,с EWG strength number of EWGs proximity of EWGS Br он он bromoacetie acid 2-dibromoacetic acid Explanation: сн, снс CH2C EWG strength number of EWGS proximity of EWGS or Он Он 3-fluoropropanoic acid 2-fluoropropanoie seid Choose the more acidic proton from each mo lecule, i.e., each molecule has two acidic protons. which is more acidic? which is more acidic? он но но Он Br Br Why? Why?
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter20: Acidity And Pka Of Phenols
Section: Chapter Questions
Problem 1E
Related questions
Question
Transcribed Image Text:13. Which acid in each pair below is the stronger acid? Please explain your answer with the structural considerations
presented in class - EWG strength, number of EWGS, or proximity of EWGS.
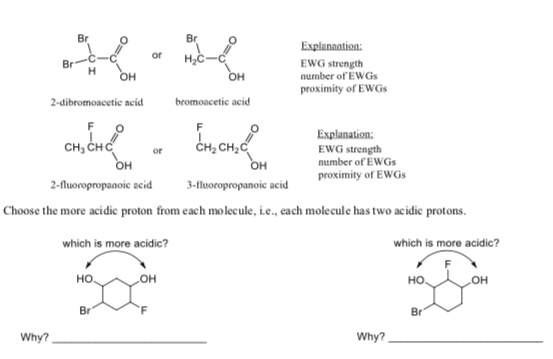
Transcribed Image Text:Br
Br
Explanaation:
or
н,с
EWG strength
number of EWGs
proximity of EWGS
Br
он
он
bromoacetie acid
2-dibromoacetic acid
Explanation:
сн, снс
CH2C
EWG strength
number of EWGS
proximity of EWGS
or
Он
Он
3-fluoropropanoic acid
2-fluoropropanoie seid
Choose the more acidic proton from each mo lecule, i.e., each molecule has two acidic protons.
which is more acidic?
which is more acidic?
он
но
но
Он
Br
Br
Why?
Why?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT