13.25 By referring to Figure 13.15, determine whether the addi tion of 40.0 g of each of the following ionic solids to 100 g of water at 40 °C will lead to a saturated solution: (a) NaNO (b) KCl, (c) K2Cr2O7, (d) Pb(NO3)2. 10 1 SECTION 13.3 Factors Affectin Henry's law Go Figure Go Figure How does the solubility of KCI at 80 °C compare with that of NaCl at the same temperature? molarity), P nality constant Between which two gases would you e this graph? s on the solute ter at 25 Cand CH 100 90 n25°Cwateris 2.0 NANO3 partial pressure °Calso doubles 70 CaCl2 CO 60 bonated bever Pb(NO3)2 nl atm. When 50 КСІ 1.0 e the solution 40 sfrom the sol NaCl 30 KСIO, Не 20 10 Ce2(SO4)3 20 10 0 Temperature 0 0 10 20 30 40 50 60 70 80 90 100 AFigure 13.16 Solubilities of four gase of temperature. The solubilities are in m solution, for a constant total pressure o Temperature (°C) A Figure 13.15 Solubilities of some ionic compounds in water as a function of temperature. Temperature Effects The solubility of most solid solutes in water increases as the solution temperature increases, as Figure 13.15 shows. There are exceptions to this rule, however, as seen for Ce2(SO4)3, whose solubility curve slopes downward with increasing temperature. In contrast to solid solutes, the solubility of gases in water decreases with increasing tem- perature (Figure 13.16). If a glass of cold tap water is warmed, you can see bubbles on the inside of the glass because some of the dissolved air comes out of solution. Similarly, as carbonated beverages are allowed to warm, the solubility of CO2 decreases, and CO2 (g) escapes from the solution. of solution when ution is reduced es in 13.4 Solubility (g of salt in 100 g H2O) KNO3 % R 8 R K2Cr2O7 Solubility (mM)
13.25 By referring to Figure 13.15, determine whether the addi tion of 40.0 g of each of the following ionic solids to 100 g of water at 40 °C will lead to a saturated solution: (a) NaNO (b) KCl, (c) K2Cr2O7, (d) Pb(NO3)2. 10 1 SECTION 13.3 Factors Affectin Henry's law Go Figure Go Figure How does the solubility of KCI at 80 °C compare with that of NaCl at the same temperature? molarity), P nality constant Between which two gases would you e this graph? s on the solute ter at 25 Cand CH 100 90 n25°Cwateris 2.0 NANO3 partial pressure °Calso doubles 70 CaCl2 CO 60 bonated bever Pb(NO3)2 nl atm. When 50 КСІ 1.0 e the solution 40 sfrom the sol NaCl 30 KСIO, Не 20 10 Ce2(SO4)3 20 10 0 Temperature 0 0 10 20 30 40 50 60 70 80 90 100 AFigure 13.16 Solubilities of four gase of temperature. The solubilities are in m solution, for a constant total pressure o Temperature (°C) A Figure 13.15 Solubilities of some ionic compounds in water as a function of temperature. Temperature Effects The solubility of most solid solutes in water increases as the solution temperature increases, as Figure 13.15 shows. There are exceptions to this rule, however, as seen for Ce2(SO4)3, whose solubility curve slopes downward with increasing temperature. In contrast to solid solutes, the solubility of gases in water decreases with increasing tem- perature (Figure 13.16). If a glass of cold tap water is warmed, you can see bubbles on the inside of the glass because some of the dissolved air comes out of solution. Similarly, as carbonated beverages are allowed to warm, the solubility of CO2 decreases, and CO2 (g) escapes from the solution. of solution when ution is reduced es in 13.4 Solubility (g of salt in 100 g H2O) KNO3 % R 8 R K2Cr2O7 Solubility (mM)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 76QAP: The freezing point of 0.10 M KHSO3 is -0.38C. Which of the following equations best represents what...
Related questions
Question

Transcribed Image Text:13.25 By referring to Figure 13.15, determine whether the addi
tion of 40.0 g of each of the following ionic solids to 100 g of
water at 40 °C will lead to a saturated solution: (a) NaNO
(b) KCl, (c) K2Cr2O7, (d) Pb(NO3)2.
10 1
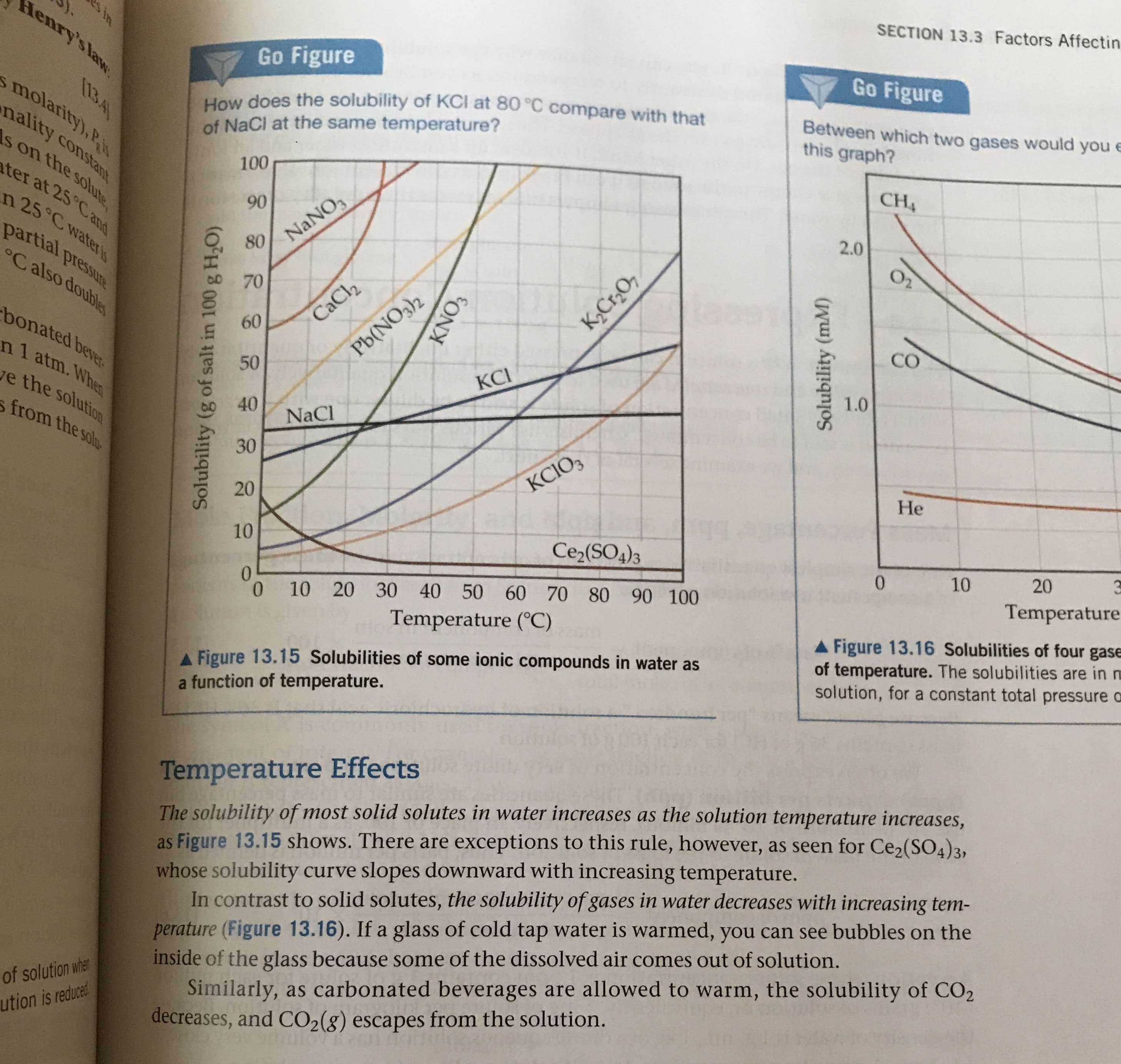
Transcribed Image Text:SECTION 13.3 Factors Affectin
Henry's law
Go Figure
Go Figure
How does the solubility of KCI at 80 °C compare with that
of NaCl at the same temperature?
molarity), P
nality constant
Between which two gases would you e
this graph?
s on the solute
ter at 25 Cand
CH
100
90
n25°Cwateris
2.0
NANO3
partial pressure
°Calso doubles
70
CaCl2
CO
60
bonated bever
Pb(NO3)2
nl atm. When
50
КСІ
1.0
e the solution
40
sfrom the sol
NaCl
30
KСIO,
Не
20
10
Ce2(SO4)3
20
10
0
Temperature
0
0
10 20 30 40 50 60 70 80 90 100
AFigure 13.16 Solubilities of four gase
of temperature. The solubilities are in m
solution, for a constant total pressure o
Temperature (°C)
A Figure 13.15 Solubilities of some ionic compounds in water as
a function of temperature.
Temperature Effects
The solubility of most solid solutes in water increases as the solution temperature increases,
as Figure 13.15 shows. There are exceptions to this rule, however, as seen for Ce2(SO4)3,
whose solubility curve slopes downward with increasing temperature.
In contrast to solid solutes, the solubility of gases in water decreases with increasing tem-
perature (Figure 13.16). If a glass of cold tap water is warmed, you can see bubbles on the
inside of the glass because some of the dissolved air comes out of solution.
Similarly, as carbonated beverages are allowed to warm, the solubility of CO2
decreases, and CO2 (g) escapes from the solution.
of solution when
ution is reduced
es in
13.4
Solubility (g of salt in 100 g H2O)
KNO3
% R 8 R
K2Cr2O7
Solubility (mM)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning