How many grams of KCIO, would be required to form a saturated solution at 70°C? 100 90 80 NANO 70 60 CaCl 50 Pb(NO)2 40 NaCI KCI 30 20 KCIO, 10 Ce,(SOJ3 0 10 20 30 40 50 60 70 80 90 100 Temperature (°C) Solubility (g of salt in 100 g H,0) FONY
How many grams of KCIO, would be required to form a saturated solution at 70°C? 100 90 80 NANO 70 60 CaCl 50 Pb(NO)2 40 NaCI KCI 30 20 KCIO, 10 Ce,(SOJ3 0 10 20 30 40 50 60 70 80 90 100 Temperature (°C) Solubility (g of salt in 100 g H,0) FONY
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 102AP
Related questions
Question
Transcribed Image Text:of Year Survey
GL- Solutions, Acids & Bases Test X
s/60a3f713a98ac02fe8247c04
HAC Home View Summary
x M Inbox (1,470) - emmaree.hernan
9 Aceable Driving | D. NG Chainsaw Dance De..
A Gloval Regents Revi.
9 10
O Weddings | Weddin...
Golf Courses Washi.
P Slit Glamorous Lace.
Free Decorative Lin.
P (1) Pinterest
f Facebook
6
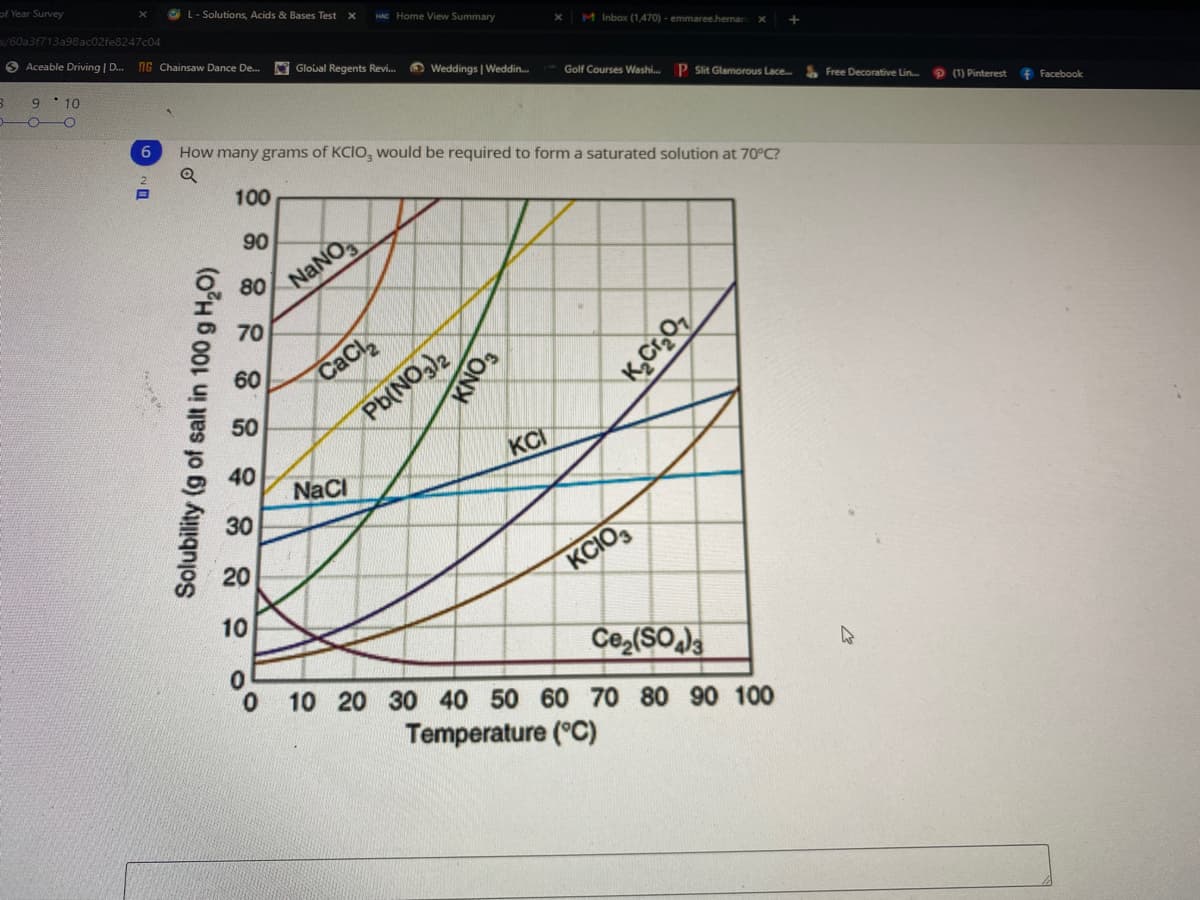
How many grams of KCIO, would be required to form a saturated solution at 70°C?
100
90
80
NANO
70
60
CaCl
50
Pb(NO)2
KCI
40
NaCi
30
20
KCIO,
10
Ce (SO
0.
10 20 30 40 50 60 70 80 90 100
Temperature (°C)
Solubility (g of salt in 100 g H,O)
GONX
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning